Human Anti-EBV-EA-D IgA ELISA Kit
Regulatory status: For research use only, not for use in diagnostic procedures.
2. Calibrator (IgA, human), ready for use, dark red, 1 x 2.0 ml .CAL.
3. Positive control (IgA, human), ready for use, blue, 1 x 2.0 ml .POS CONTROL.
4. Negative control (IgA, human), ready for use, green, 1 x 2.0 ml .NEG CONTROL.
5. Enzyme conjugate, peroxidase-labelled anti-human IgA (rabbit), ready for use, orange, 1 x 12 ml .CONJUGATE.
6. Sample buffer, ready for use, light blue, 1 x 100 ml .SAMPLE BUFFER.
7. Wash buffer, 10x concentrate, colourless, 1 x 100 ml .WASH BUFFER 10x.
8. Chromogen/substrate solution, TMB/H2O2, ready for use, colourless, 1 x 12 ml .SUBSTRATE.
9. Stop solution, 0.5 M sulphuric acid, ready for use, colourless, 1 x 12 ml .STOP SOLUTION.
10. Test instruction --- 1 booklet
11. Quality control certificate --- 1 protocol
For every group of tests performed, the extinction readings of the calibrator and the ratios of the positive and negative controls must lie within the limits stated for the relevant test kit lot. A quality control certificate containing these reference values is included. If the values specified for the controls are not achieved, the test results may be inaccurate and the test should be repeated.
The binding activity of the antibodies and the activity of the enzyme used are temperature-dependent. It is therefore recommended using a thermostat in all three incubation steps. The higher the room temperature (+18°C to +25°C) during the incubation steps, the greater will be the extinction. Corresponding variations apply also to the incubation times. However, the calibrator is subject to the same influences, with the result that such variations will be largely compensated in the calculation of the result.
Antigen: The microplate wells were coated with the recombinant Epstein-Barr virus early antigen diffuse. The protein was expressed in E. coli and the molecular weight is 45 kDa.
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
Human herpesvirus is a type of large, enveloped double-stranded DNA virus that belongs to the family Herpesviridae. Human herpes viruses infect a wide range of hosts and mainly damage mucous membranes, skin and nerve tissues. Currently, more than 100 subtypes have been discovered. The most studied human herpes viruses include: human cytomegalovirus, Epstein-Barr virus(EBV), varicella-zoster virus, and herpes simplex virus. Epstein-Barr virus is the first human tumor virus discovered and is a Class I carcinogen declared by WHO. It is associated with post-transplantation lymphoproliferative disease, Hodgkin lymphoma (HL), Burkitt lymphoma, nasopharyngeal carcinoma, EBV-related gastric cancer and other malignant tumors are related to the occurrence of malignant tumors, and it has the potential to be used as a prognostic predictor and therapeutic target. EBV belongs to Humanherpesvirus 4 (HHV-4) and is a member of the gammaherpesvirus subfamily. Its morphological structure is similar to other herpesviruses. It is spherical in shape with a diameter of 180 nm and includes a core, capsid, and envelope. The nuclear sample contained a genomic double-stranded DNA with an average length of 175 kb, encoding more than 85 proteins. The capsid consists of 162 shell particles forming an icosahedron structure. The envelope of EBV is composed of the nuclear membrane of the host cell, and a variety of virus-encoded membrane proteins are expressed on the membrane. It is currently believed to include at least 10 glycoprotein, including gp350, gB, gH, gL, gp42, gM, gN, gpl50, gp78, and gp55. Gp350 is the most important glycoprotein on the surface of EBV. In the early stage of infection, the virus binds to CR2/CD21 molecules on the surface of B cells through gp350, promoting the infection of B cells. Early research found that the isolated gp350 protein can stimulate human B cells to produce neutralizing antibodies against EB virus and weaken the virus's ability to infect. gp350 is the main research target for the study of EB virus preventive vaccines. gH, gL and gp42 exist in the form of heterotrimers on the envelope of EB virus and mainly mediate the fusion process of EB virus and B cells, while the heterodimer composed of gH and gL mainly mediates the fusion process of EB and epithelial cells. Since gH, gL and gp42 trimers are degraded after binding to MHC class II molecules, which are present in the endoplasmic reticulum of B cells but not expressed in epithelial cells, EBV produced from epithelial cells contains more gH, gL and gp42 trimers, but less in lymphocyte-derived EBV.
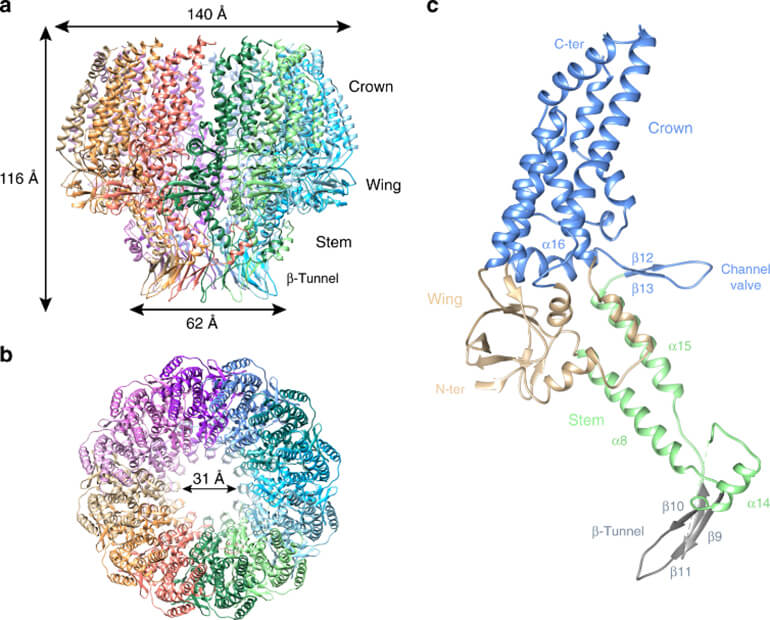 Figure 1. Structural characterization of EBV portal.(Source: Machón C, et al. 2019)
Figure 1. Structural characterization of EBV portal.(Source: Machón C, et al. 2019)
When EBV infects a host cell, the viral DNA will exist in the form of a cyclic episome in the host cell nucleus. The virus combines with the free cyclic episome through its expressed nucleoprotein to jointly maintain and regulate the protein expression of the virus and the host cell. The number of characteristic repeat sequences of the virus on this DNA episome is consistent with that of the parent virus strain. Therefore, by identifying the number of repeat sequences in the episome, it can be determined whether the source of EB virus in the cell is consistent. During the initial infection, EBV infects the epithelial cells of the oropharynx with very low efficiency through saliva transmission and other channels, and cleaves and replicates within the cells to synthesize mature virus particles. A part of the virus particles infects initial B cells through transcytosis in the epithelial cell layer. Under the interaction of EBV surface membrane protein gp350/220 and B cell surface receptor CD21, the EBV capsule and cell membrane fuse and EBV is endocytosed into the cell. After being transported to the vicinity of the nucleus, the nucleocapsid fuses with the nuclear membrane, and the EBV linear genome enters the nucleus. Under the mediation of the terminal repeats of the EBV genome, it circularizes to form a free body and stably exists in the nucleus, thereby establishing latent infection and inducing host B cells differentiate into memory cells and replicate along with their cell cycle. At this time, EBV only expresses a limited number of genes to maintain its latent infection state and host cell proliferation. When stimulated by certain conditions, such as hypoxia, DNA damage, etc., memory B cells differentiate into effector cells, inducing the transition of EBV from a latent infection state to a lytic infection state. During this process, the EBV genome replicates in large quantities to form a linear EBV genome concatemer, and fragmented at the TR to form a single genome, which is then further packaged to form infectious mature virus particles and released outside the cell. Mature EBV particles can shuttle between B lymphocytes and epithelial cells to complete the infection cycle, and can be re-transmitted from nasopharyngeal epithelial cells through saliva. EBV adopts strategies to regulate these signaling pathways at different levels to minimize host antiviral activity and thereby maintain persistent infection. Among them, EBV has developed a series of strategies to escape host immunity during the latent infection and lytic infection stages to ensure the continuation of the viral genome and produce a large number of mature virus particles at the appropriate time to further expand the infection. After EBV infects B cells, they can be transformed into immortal lymphoblastoid cell lines (LCL). The transformed LCL becomes the type III latent infection state of EBV and can last for several months, which plays an important role in studying the early steps of latent virus infection. EBV can also transform EBV-infected tumor cells and, together with LCL, serves as an important tool for EBV latent infection and epigenetic regulation.
Human Herpesvirus 4 (HHV-4)
Herpesvirus 4
Infectious Mononucleosis Virus
References
1. Machón C, et al. Atomic structure of the Epstein-Barr virus portal. Nat Commun. 2019,10(1):3891.
Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors
J Clin Pathol
Authors: Kerr JR.
Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis
Med Microbiol Immunol.
Authors: Yin H, Qu J, Peng Q, Gan R.
Invoice / Purchase Order
Credit card
![]()