Gangliosides - sialic acid-bearing glycolipids - are major cell surface determinants on neurons and axons. The same four closely related structures, GM1, GD1a, GD1b, and GT1b, comprise the majority of total brain gangliosides in mammals and birds. Gangliosides regulate the activities of proteins in the membranes in which they reside and also act as cell-cell recognition receptors.
Distribution Pattern
Hundreds of gangliosides have been identified in vertebrate cells. Knowledge of their tissue distribution is required for understanding the functions of major brain gangliosides. The main gangliosides (~95%) of adult mammalian brain are ganglio series GM1, GD1a, GD1b, and GT1b, while lactosyl series gangliosides such as GM3 are found mainly in the extra-neural tissues. The remaining ~5% of minor components include gangliosides GM4, GM3, GD3, GM2, GD2, Fuc-GM1, Fuc-GD1b, GT1a and GP1c, the relative proportions of which vary depending on species. More detailed knowledge of their distribution may help direct future functional studies.
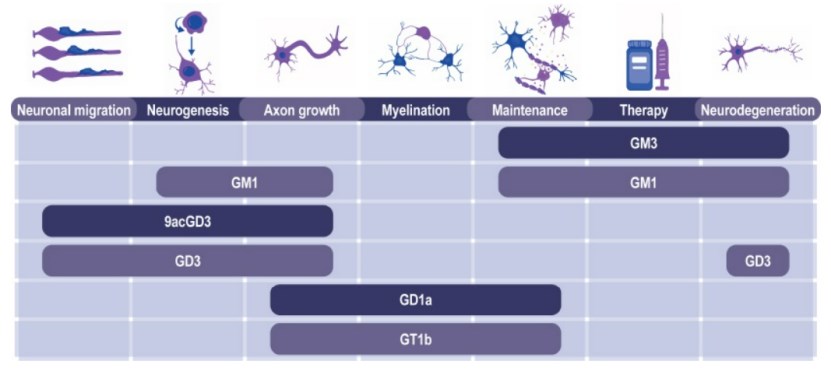 Figure 1. Ganglioside functions in nervous system development, function, and repair
Figure 1. Ganglioside functions in nervous system development, function, and repair
Clinical significance
Neurodegenerative disease
The importance of gangliosides is highlighted in severe neurodegenerative disorders, including Huntington's disease (HD), Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), stroke, multiple sclerosis, and epilepsy. In HD, PD, and in some forms of epilepsy, experimental evidence strongly suggests a potential role of gangliosides in disease pathogenesis and treatment. Clinical experience is greatest with GM1in the treatment of stroke. GM1 administration also results in decreased levels of mutant huntingtin, and profound disease-modifying effects for HD. GD1b may be a target molecule for autoantibodies in some patients with acute sensory ataxic neuropathy. Anti-GD1b has been found in some patients with Guillain–Barré syndrome (a disorder affecting the peripheral nervous system) and may contribute to the pathogenesis of sensory disturbance and demyelination.
Cancer and therapy
Several gangliosides are found to have elevated expressions in tumor cells. Diagnostics biomarkers and therapeutic treatments against tumors are being investigated using antibodies to target these gangliosides. Anti-GA1 has been shown to eliminate natural killer (NK) cell activity, which is being pursued as an anti-immunogenic treatment for use in bone marrow transplants and other similar applications. Lymphoma has been shown to have an increased level of asialo GM2 and passive immunization with monoclonal immunoglobulin G3 antibodies to asialo GM2 has effectively suppressed tumor formation in these cells. GD3 expression is unusually high in basal cell carcinomas and malignant melanomas. It has been investigated for immune targeting human melanoma cells with anti-GD3. GM3 is not expressed in melanocytes normally, but it is detected in 60% of primary melanomas and in 75% of metastatic melanomas. It is also elevated in the serum of patients with breast cancer and may be a biomarker for the disease, while disialylated gangliosides GD2 and GD3 may be markers of neuroectoderm origin in neuroblastoma.
Autoimmune diseases
Gangliosides are significant roles in protecting against immune attacks, yet they can become targets for autoimmunity and act as receptors for microbes (like the influenza viruses) and toxins (such as the cholera toxin). Asialo-GM2 has been identified as a receptor for many types of bacteria, including Neisseria gonorrhoeae and Haemophilus influenza. Antibodies against gangliosides (AGA) are important, especially in acquired demyelinating immune-mediated neuropathies, like Guillain–Barré syndrome (GBS) and its variant, the Miller–Fisher syndrome (MFS).
Creative Diagnostics developed a portfolio of gangliosides antibodies for the research of gangliosides functions, disease pathogenesis, and potential treatment.
Highlights
- High Affinity and specificity.
- Broad applications covered: ELISA, IHC, FC, ICC, WB, IF, and TLC ("Far Eastern") blotting.
- Well validated with abundant quality control data.
- Matched standards available.