Human Epstein-Barr Virus EBNA-1 IgG ELISA Kit
Regulatory status: For research use only, not for use in diagnostic procedures.
2. Standard serum (ready-to-use) STD, Human serum in phosphate buffer with protein, negative for anti-HIV-Ab, HBs-Ag (Hepatitis B-Virus-surface antigen) and anti-HCV-Ab, preservative: <0.1% sodium azide colouring: Amaranth O. 2 × 2 mL
3. Negative control serum (ready-to-use) NEG, Human serum in phosphate buffer with protein, negative for anti-HIV-Ab, HBs-Ag (Hepatitis B-Virus-surface antigen) and anti-HCV-Ab, preservative: <0.1% sodium azide colouring: Lissamine green V. 2 mL
4. Anti-human-IgG-conjugate (ready-to-use) APC, Anti-human-IgG-IgM from goat (polyclonal), conjugated to alkaline phosphatase,
stabilized with protein stabilization solution preservative: 0.01 % methylisothiazolone, 0.01 % bromnitrodioxane. 13 mL
5. Washing solution concentrate (sufficient for 1000 mL) WASH, Sodium chloride solution with Tween 20, 30 mM Tris preservative: <0.1% sodium azide. 33.3 mL
6. Dilution buffer: Phosphate buffer with protein and Tween 20; preservative: <0.1% sodium azide 0.01 g/l Bromphenol blue sodium salt. 2 × 50 mL
7. Stopping solution STOP, 1.2 N sodium hydroxide. 15 mL
8. Substrate (ready-to-use) pNPP, Para-nitrophenylphosphate, solvent free buffer preservative: <0.1 % sodium azide (Substrate in unopened bottle may have a slightly yellow coloring. This does not reduce the quality of the product!). 13 mL
2. Control sera / Standard sera: After opening at 2-8°C. Until expiry date, 24 months after date of production.
3. Conjugate: Ready-to-use solution, at 2-8°C. Avoid contamination (sterile tips!). Until expiry date, 28 months after date of production
4. Dilution buffer: After opening at 2-8°C (Discard cloudy solutions!), 24 months. Unopened, until expiry date, 36 months after date of production
5. Washing solution: Concentrate after opening at 2-8°C, until expiry date. Working dilution at 2-8°C, 2 weeks. Working dilution at room temperature, 1 week. Bottles used for the working dilution should be cleaned regularly, discard cloudy solutions
6. Substrate: Ready-to-use solution at 2-8°C, protected from light! Avoid contamination (sterile tips!) Discard when solution turns yellow (extinction against distilled water. > 0.25). Until expiry date, 24 months after date of production
7. Stopping solution: After opening at room temperature, until expiry date.
EBV transmission takes place primarily via the saliva of infected individuals. Transfer of the virus via blood, blood products and bone marrow transplants have also been reported, but this mode of transmission is comparatively rare.
On primary infection, the cells of the salivary gland are infected first. At this stage of infection respiratory symptoms are very common. Due to the subsequent infection of B cells in the adjacent lymphoid tissue, the virus spreads throughout the body. Infection of B cells results in a polyclonal stimulation of lymphoproliferation which is normally controlled by the immune system. In the later course of infection, high fever, splenomegaly, lymphadenitis, thrombocytopenia and hepatitis may be observed. The disease resulting from primary infection is called infectious mononucleosis (IM) or glandular fever. Since viral transmission principally happens by oral contact, the primary infection has also been called "kissing disease". In rare cases acute mononucleosis may progress to a chronic disease, and reactivation of EBV has been observed in immunosuppressed patients.
EBV is closely associated with nasopharyngeal carcinoma, and Burkitt lymphoma (BL), is at least partly correlated with EBV. This B cell tumor of monoclonal origin is endemically clustered in tropical regions of Africa and Asia. The geographical distribution of BL correlates with that of malaria. Since it is believed that infections with Plasmodium have some influence on the immune system, it is suggested that malaria might be an important cofactor for the development of Burkitt lymphoma in these regions.
Due to the complex structure of the virus, the tight regulation of the viral life cycle, and the appearance of latent or productive infections, the antibody response seen after EBV infection may be quite complex and therefore difficult to interpret.
EBV transmission takes place primarily via the saliva of infected individuals. Transfer of the virus via blood, blood products and bone marrow transplants have also been reported, but this mode of transmission is comparatively rare.
On primary infection, the cells of the salivary gland are infected first. At this stage of infection respiratory symptoms are very common. Due to the subsequent infection of B cells in the adjacent lymphoid tissue, the virus spreads throughout the body. Infection of B cells results in a polyclonal stimulation of lymphoproliferation which is normally controlled by the immune system. In the later course of infection, high fever, splenomegaly, lymphadenitis, thrombocytopenia and hepatitis may be observed. The disease resulting from primary infection is called infectious mononucleosis (IM) or glandular fever. Since viral transmission principally happens by oral contact, the primary infection has also been called "kissing disease". In rare cases acute mononucleosis may progress to a chronic disease, and reactivation of EBV has been observed in immunosuppressed patients.
EBV is closely associated with nasopharyngeal carcinoma, and Burkitt lymphoma (BL), is at least partly correlated with EBV. This B cell tumor of monoclonal origin is endemically clustered in tropical regions of Africa and Asia. The geographical distribution of BL correlates with that of malaria. Since it is believed that infections with Plasmodium have some influence on the immune system, it is suggested that malaria might be an important cofactor for the development of Burkitt lymphoma in these regions.
Due to the complex structure of the virus, the tight regulation of the viral life cycle, and the appearance of latent or productive infections, the antibody response seen after EBV infection may be quite complex and therefore difficult to interpret.
The ELISA Epstein-Barr Virus EBNA 1 IgG may be used as quantitative and qualitative tests for detection of human anti-Epstein-Barr Virus antibodies in serum or plasma. For Research Use Only. Not for use in diagnostic procedures.
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
Nine human herpesviruses have been confirmed, belonging to the three subfamilies of a, beta, and gamma. Among them, Epstein-Barr virus (EBV) belongs to the human gamma herpesvirus subfamily. EBV is the most common and persistent virus in humans, and about 95% of the world's people are asymptomatic carriers of it. As a double-stranded DNA virus, EBV has a genome of about 175kb containing about 80 open reading frames and 44 non-coding RNAs. EBV mainly infects B lymphocytes and epithelial cells. It can not only persist throughout life, but also has alternating latent and lytic replication cycles. Usually, individuals have basically no symptoms after infection, which is a latent infection. However, in a few cases, especially when adolescents are infected with EBV for the first time, it can cause acute infectious mononucleosis. In immunosuppressed individuals such as AIDS patients and organ transplant recipients, because the immune system loses control of EBV replication, EBV begins to enter the lytic phase, causing fatal lymphoproliferative diseases. In addition, EBV latent infection is associated with the occurrence of many lymphocyte-derived and epithelial-derived tumors, including Burkitt lymphoma, Hodgkin lymphoma, NK/T cell lymphoma and gastric cancer, among which EBV latent type I infection is the main pathogenic factor for nasopharyngeal carcinoma. However, at present, no clinical means have been found to effectively prevent or eliminate EBV infection, or to block or interfere with the life cycle of EBV. The life cycle of EBV is mainly divided into five different stages: EBV invasion, infection of B cells, proliferation, differentiation and continued survival, of which the last four stages are related to EBV disease. EBV is transmitted through saliva, invades the epithelial tissue of the oropharyngeal mucosa, and then infects immature B cells. Except for some viruses that enter the lytic replication cycle, the rest of EBV will enter the latent stage III. In order to activate B cells and drive their proliferation and transformation, EBV after infecting immature B cells will express all latent genes including EBV nuclear antigen (EBNA), latent membrane protein (LMP), small RNA (EBER) and microRNA. However, due to their high immunogenicity, infected cells in the latent phase I will be quickly cleared by EBV-specific T lymphocytes. In order to continue to survive effectively in B cells, EBV begins to enter the latent phase 1 and evades immunity by downregulating the expression of multiple immunogenic proteins. At this time, EBV only expresses three viral proteins: EBNA-I, LMP1, and LMP2A. In the subsequent proliferation process, B cells are induced to differentiate into memory B cells by CD40/BCR receptor signals simulated by LMP1 and LMP2A, including split memory B cells and resting memory B cells. In the latent phase I, along with the division of memory B cells, EBV only expresses EBNA1 protein to ensure that the viral genome is replicated and distributed with the host cells. In the latent phase 0, after the memory B cells infected with EBV enter the peripheral blood, they will maintain immune silence by stopping the gene expression of all viral proteins. At this time, the resting memory B cells carrying EBV will not be attacked by the host immune system. Therefore, resting memory B cells are very likely to be the site where EBV exists for a long time. Finally, in some cases, memory B cells can also terminally differentiate into plasma cells and secrete antibodies. EBV whose lytic program is reactivated will enter the lytic cycle again. At the same time, as plasma cells secrete antibodies, infectious EBV is released and infects epithelial cells. EBV can proliferate in large numbers in epithelial cells, and the offspring viruses are transmitted to other hosts through saliva.
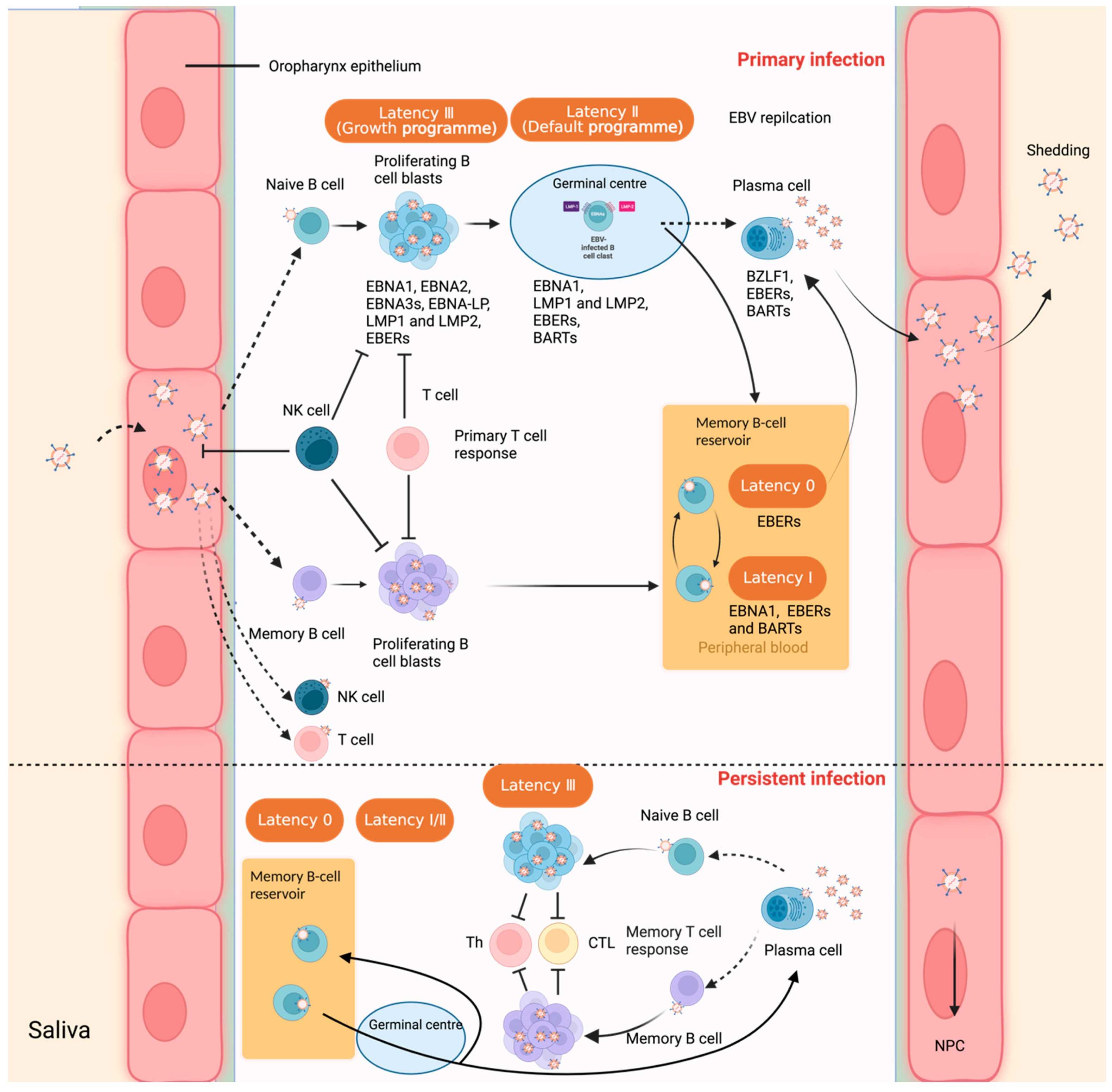 Figure 1. Interaction between Epstein–Barr virus and the human host, and virus latent infection in B lymphocytes. (Sources: Yu H, et al. 2023)
Figure 1. Interaction between Epstein–Barr virus and the human host, and virus latent infection in B lymphocytes. (Sources: Yu H, et al. 2023)
As the only viral protein consistently expressed in all EBV-related tumors, EBNA1 plays a key role in the maintenance, replication and segregation of the EBV genome, as well as viral gene expression and host cell survival. At the same time, EBNA1 is a virus-encoded protein and has no orthologs in known host cells. Therefore, EBNA1 is considered to be an important potential drug target for therapeutic intervention in EBV-related cancers. The crystal structure of EBNA1 (470-607) is a dimer structure composed of two monomers. At the same time, the dimer structure of EBNA1 (470-607) includes the core domain EBNA1 (s04-604) and the flanking domain EBNA1 (77-490). The core domain EBNA1 (504-604) contains an eight-strand antiparallel "β barrel" structure, which is composed of four β strands in each monomer, and the β strands in each monomer are connected by two a helices on the outside of the "β barrel". The flanking domain EBNA1 (77-90) forms an a-helix that is almost parallel to the non-crystallographic axis, close to the two a-helices in the core domain, and connected to the first beta strand of the "beta barrel" through an extended loop. EBNA1 is the only viral protein that is consistently expressed in all EBV-related tumors. It can specifically bind to the DNA sequence of the viral origin of plasmid replication (OriP), which is essential for viral DNA replication and the maintenance of the EBV genome during the proliferation of latently infected cells. OriP contains two functional elements: the binary symmetry (DS) element and the repeat sequence (FR). The DS element is the replication origin in oriP. Throughout the cell cycle, EBNA1 binds to the DS element and replicates the EBV genome with the help of host cell functional proteins. When bound to the oriPFR element, EBNA1 can act as a transcriptional activator to enhance the expression of reporter genes on the plasmid containing FR in a distance-independent manner. In addition, the mitotic segregation of the EBV episomal genome also requires the help of two viral components, EBNAI and oriPFR elements. The EBV episomal genome is attached to the host cell chromatin through EBNAI to ensure the stable segregation of viral DNA during cell mitosis. EBV requires the binding of EBNA1 dimers to oriP recognition sequences in gene replication, transcription activation, and control of the stable segregation of the episomal genome during cell division. The DNA binding domain of EBNAI is located in the protein portion containing amino acids 459-607.
EBV Nuclear Antigen 1
EBNA1
Epstein-Barr Virus Nuclear Antigen 1
References
1. Yu H, Robertson ES. Epstein-Barr Virus History and Pathogenesis. Viruses. 2023, 15(3):714.
2. Damania B, et al. Epstein-Barr virus: Biology and clinical disease. Cell. 2022, 185(20):3652-3670.
Biomarkers for PTLD diagnosis and therapies
PEDIATRIC NEPHROLOGY
Authors: Martinez, Olivia M.
Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) in Oropharyngeal Cancer Associated with EBV and HPV Coinfection
VIRUSES-BASEL
Authors: Strycharz-Dudziak, Malgorzata; Foltyn, Sylwia; Dworzanski, Jakub; Kielczykowska, Malgorzata; Malm, Maria; Drop, Bartlomiej; Polz-Dacewicz, Malgorzata
Invoice / Purchase Order
Credit card
![]()