Recombinant Alpaca Anti-Rabbit IgG monoclonal antibody, Alexa Fluor®488
Alpaca Anti-Rabbit IgG monoclonal antibody, Alexa Fluor®488 for IF, WB, ELISA, recombinant VHH
Immunofluorescence 1:1,000
Super-resolution microscopy 1:1,000
Western blot 1:1,000
Optimal working concentration is application-dependent and should be determined by testing a dilution range from 1:250 to 1:2,000.
Note: Image acquisition time may have to be optimized.
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
IgG is one of the most abundant proteins in human serum, accounting for about 10-20% of plasma proteins. It dominates the five immunoglobulin categories (IgM, IgD, IgG, IgA, and IgE). Not only is IgG an essential part of the human adaptive immune system, but it's also wildly varied. It contains between 82-96% protein and 4-18% carbohydrate, and it has two chains and two light chains attached by disulfide bonds in the typical "H" shape of antibodies. This further separates IgG into four subclasses: IgG1, IgG2, IgG3 and IgG4. While the amino acids in question are 90 per cent identical, their function and structure (antigen-binding, immunocompetent formation, complement synthesis, half-life) varies among these subclasses. The most dominant subclass is IgG1, which responds mostly to soluble protein and membrane antigens. This subclass functions in detoxifying toxicants, inhibiting pathogen invasion, and activating antibody-dependent cellular cytotoxicity (ADCC). IgG2 specifically targets polysaccharide antigens and, without it, the immune system is less effective against bacterial capsular polysaccharides, making it vulnerable to encapsulated bacteria. IgG3's longer hinge region confers greater pro-inflammatory and complement activation potential, while retaining a shorter half-life that inhibits inflammatory response over-extrusion. IgG4 is produced in response to antigen stimulation, often for prolonged or recurring periods, and is associated with tolerance or non-infectious immune reactions, such as in immunotherapy and some parasitic infections.
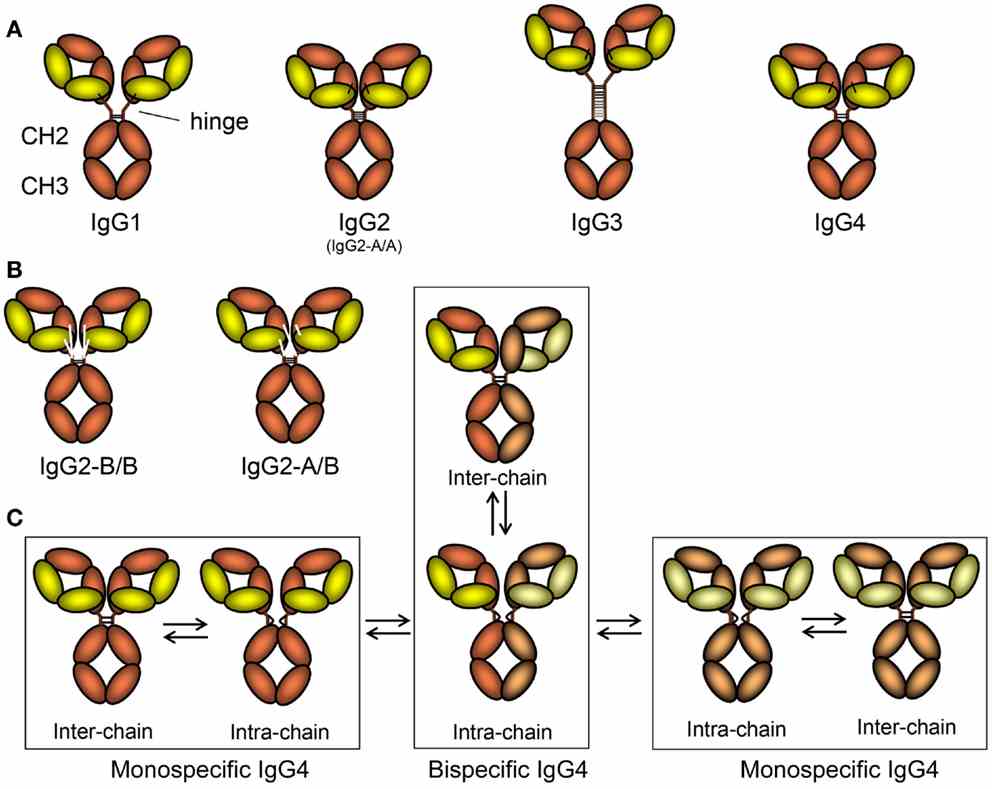 Figure 1. Schematic of IgG Subclasses and Their Isoforms. (Source: Vidarsson G, et al., 2014)
Figure 1. Schematic of IgG Subclasses and Their Isoforms. (Source: Vidarsson G, et al., 2014)
IgG antibodies not only play a role in adaptive immunity but also participate in various innate immune functions. This is because its Fc region can attach to complement C1q and Fcγ receptors (FcγR), which starts phagocytosis, cytotoxicity, and complement activation. Furthermore, IgG facilitates placental and mucosal transport by prolonging its half-life via the neonatal Fc receptor (FcRn). This highly specialized Fc structure endows IgG with broad immunoregulatory capabilities. Abnormalities or deficiencies in IgG function are often associated with various diseases. Others develop selective IgG subclass deficiencies that could lead to diminished protection against certain pathogens or antigens. IgG2 deficiency, for instance, causes heightened vulnerability to encapsulated bacterial infection; and IgG4 deficiency, although rare, impairs tolerance-mediated immune defenses. Conversely, highly elevated IgG4 levels are a hallmark of IgG4-related disease (IgG4RD), a family of multiple-organ chronic inflammatory disorders such as autoimmune pancreatitis, Mikulicz disease and interstitial nephritis. We don't fully understand how IgG4RD can happen, but high IgG4 levels could be caused by tolerance-driven immune response induced by continued antigen stimulation. Certain pathogen infections are also associated with IgG subclass distribution. For example, viral infections often elicit IgG1 and IgG3 responses, which play significant roles in neutralizing pathogens and initiating inflammation. The shorter half-life of IgG3 eliminates pathogens rapidly but requires IgG1 to create lasting memory. Moreover, IgG4 has been shown to be part of the immune response to some parasitic infections and this response is typically accompanied by high IgG4 and few symptoms.
Because of its versatility, IgG can be used to conduct therapeutic antibody research. By using monoclonal antibody technology, researchers could now design IgG antibodies that attack an antigen to cure cancer, infection and autoimmune disease. For instance, IgG1 (because of its cytotoxicity and complement-dependent cytotoxicity) is the mainstay of cancer immunotherapy. IgG4, which exerts a modest immune effect, is well-suited for treatment that targets inflammation or induces immune tolerance, like rheumatoid arthritis and multiple sclerosis. Moreover, IVIG, an immunomodulatory drug with a broad spectrum, has been used to treat various conditions such as primary immunodeficiency, Kawasaki disease and immune thrombocytopenia, with IgG as the principal agent. IgG is also a vital component of vaccines where the balancing of subclasses is key to the construction of potent vaccines. Viral vaccines, for example, are sometimes designed to stimulate the activity of IgG1 and IgG3 for immediate, long-lasting immunity. Recently, engineering of the Fc region has created new opportunities to generate next-generation IgG antibodies. This allows the immune effector activity of antibodies to be carefully controlled by changing glycosylation profiles or Fc structures. IgG, in sum, by virtue of its central position and diversity not only ensures immune balance in normal humans but also serves as a useful marker and therapeutic tool for diseases. Then, over time, as more and more is learned about its structure and function, IgG's role in precision medicine will be even more real.
Alpaca Anti-Rabbit IgG Monoclonal Antibody
Alpaca Anti-Rabbit IgG
Recombinant Alpaca Anti-Rabbit IgG Clone
References
1. Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front Immunol. 2014;5:520.
Programmed death ligand-1 induction restrains the cytotoxic T lymphocyte response against microglia
GLIA
Authors: Chauhan, Priyanka; Hu, Shuxian; Prasad, Sujata; Sheng, Wen S.; Lokensgard, James R.
Rapid Collection of Human Rectal Secretions Using OriCol Devices Is Suitable for Measurement of Mucosal Ig without Blood Contamination
JOURNAL OF IMMUNOLOGY
Authors: Czartoski, Julie; Lemos, Maria P.; Fong, Youyi; Mize, Gregory J.; Konchan, Anne; Berger, David; Maenza, Janine; McElrath, M. Juliana
- Immunoglobulin
- Nano Secondary Antibodies
- Study on the Correlation Between IgG Subtype of Anti-dsDNA Antibody and SLE
Invoice / Purchase Order
Credit card
![]()
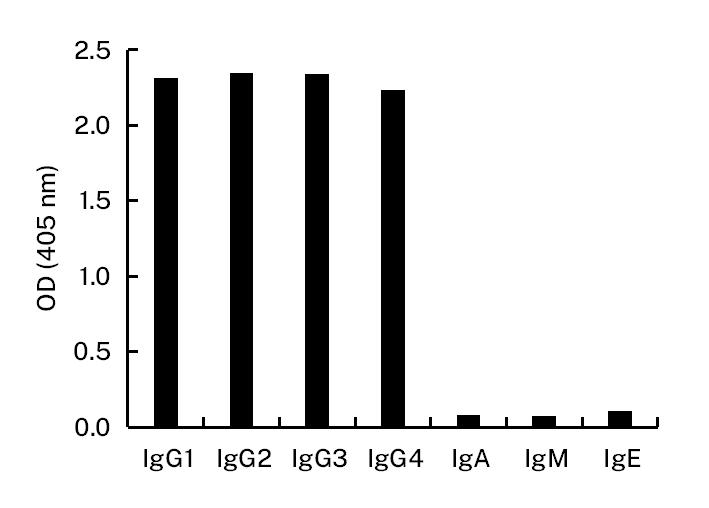
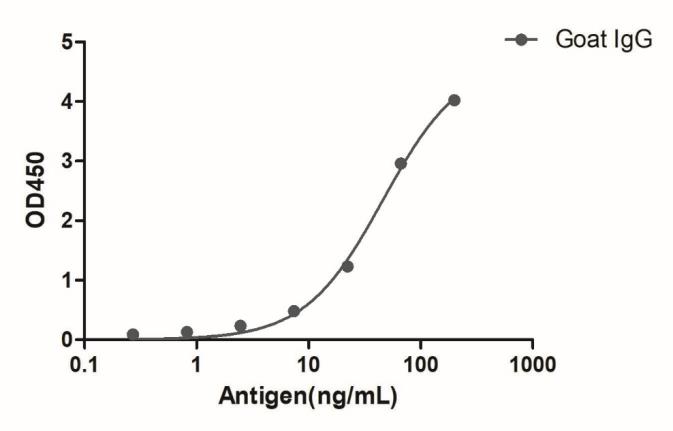
![HiResNb™ Anti-Goat IgG(Fcγ Fragment specific) VHH antibody, clone 165-212 [Biotin] (DMABB-JP05)](jpg/dmabb-jp05-1.jpg)
![HiResNb™ Anti-Goat IgG(Fcγ Fragment specific) VHH antibody, clone 165-212 [HRP] (DMABB-JP06)](jpg/dmabb-jp06-1.jpg)
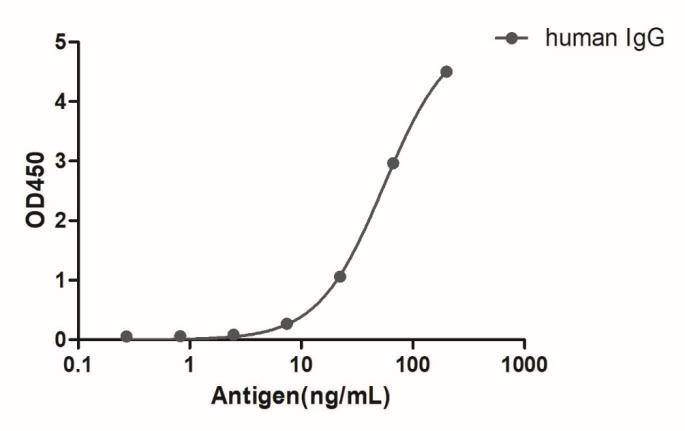
![HiResNb™ Anti-Rabbit IgG VHH antibody, clone 21F21 [Atto 488] (DMABB-JP101)](jpg/dmabb-jp101_1.jpg)
![HiResNb™ Anti-Human IgG(Fcγ fragment specific) VHH antibody, clone 134-212 [Biotin] (DMABB-JP12)](jpg/dmabb-jp12-1.jpg)
![HiResNb™ Anti-Human IgG(Fcγ fragment specific) VHH antibody, clone 134-212 [HRP] (DMABB-JP13)](jpg/dmabb-jp13-1.jpg)