Chemiluminescent Immunoassay Development
Chemiluminescent Immunoassays (CLIAs) have revolutionized biological and chemical detection. Based on the detection of a protein that glows or emits light in a chemiluminescent reaction, CLIA is a faster assays technique that does not require stop reactions and has short incubation times. In addition, it has a much wider detection range than either RIAs or ELISAs and is also extremely sensitive under low analyte concentration conditions.
CDSimple™ Chemiluminescent ELISA platform offers a seamless process through which we will develop high-quality assays for your application. Depending on your need, assays may be developed for basic research purposes or designed to include additional qualification and validation.
Key Benefits
- Antibody matched pair screening experience
- Extensive assay development experience
- Facilitate the transition from RUO to IVD
- Personalized assay development and support every step of the way
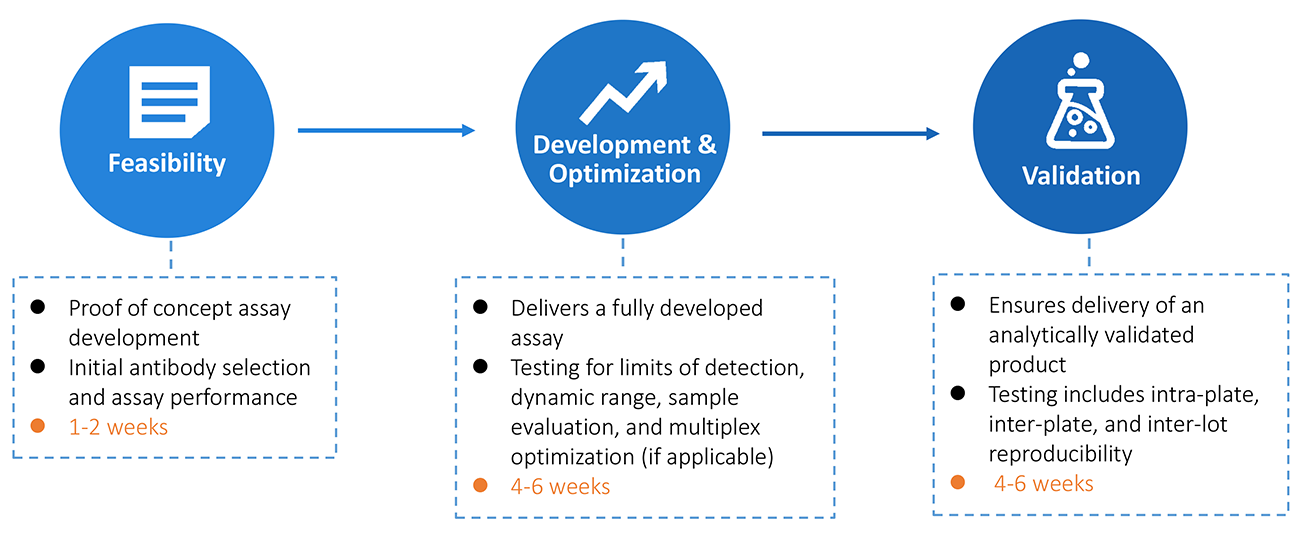
Delivery
Creative Diagnostics will carry out the approval proposal and deliver the expected results and documents in a time and cost effective manner. Our staff scientists follow rigorous guidelines for quality control with a focus on thorough optimization and validation of every aspect of assay development, including antibody specificity, assay sensitivity, reproducibility (intra- and inter-assay), cross-reactivity, standard curve range, assay accuracy (linearity and recovery) and kit stability.
