Antibody-drug conjugates (ADCs) are regarded as a novel class of powerful therapeutic agents for cancer therapy, which consist of a monoclonal antibody (mAb) covalently bound with a cytotoxic drug through a chemical linker. mAb recognize antigens overexpressed on the surface of tumor cells, and cytotoxic payload is responsible for the execution of tumor cell killing. Monomethyl auristatin E (MMAE) is the most widely used payload in ADC design. MMAE is a potent antimitotic drug that exerts its action by blocking the tubulin polymerization process resulting in cell cycle arrest and apoptosis. It has shown potent activity in pre-clinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. In International Nonproprietary Names for mAb-MMAE-conjugates, the name vedotin refers to MMAE plus its linking structure to the antibody. As of 2021, three MMAE-based ADCs have been licensed by the U.S. Food and Drug Administration (FDA).
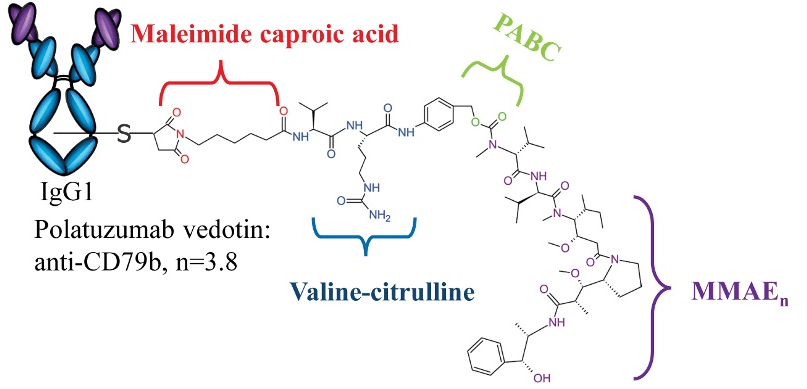 Fig. 1 Structure of Polatuzumab vedotin
Fig. 1 Structure of Polatuzumab vedotin
(Dean AQ, et al. 2021)
Table 1 Three FDA Approved MMAE-based ADCs
| ADC | Trade Name | Approval Year | Target | Antibody | Linker |
| Brentuximab vedotin | Adcetris® | 2011 | CD30 | Chimeric IgG1 | Valine-citrulline |
| Polatuzumab vedotin | Polivy® | 2019 | CD79b | Humanized IgG1 | Valine-citrulline |
| Enfortumab vedotin | Padcev® | 2019 | Nectin-4 | Human IgG1 | Valine-citrulline |
ADCs are complex molecules with unique critical quality attributes. Considering the heterogeneity and complex changes in ADC concentration and composition after ADC administration, three analytes are routinely measured in order to characterize the PK properties of an ADC: antibody-conjugated MMAE (acMMAE), total antibody, and unconjugated MMAE. Creative Diagnostics has developed anti-MMAE antibodies and paired conjugates suitable for ADC ELISA assay development. It can be used in the quantitative determination of antibody-MMAE-conjugate level in test sample for pre-clinical and clinical pharmacology study of MMAE ADC.
Bioactivity & Stability
| Coating | MMAE [BSA] (#DAG603S), 0.5 ug/ml |
| Sample | Anti-MMAE monoclonal antibody, clone 3F3 (#CABT-B8992), 0-250 ng/ml |
| Detection | Rabbit anti-Mouse IgG-HRP, 1:4,000 |
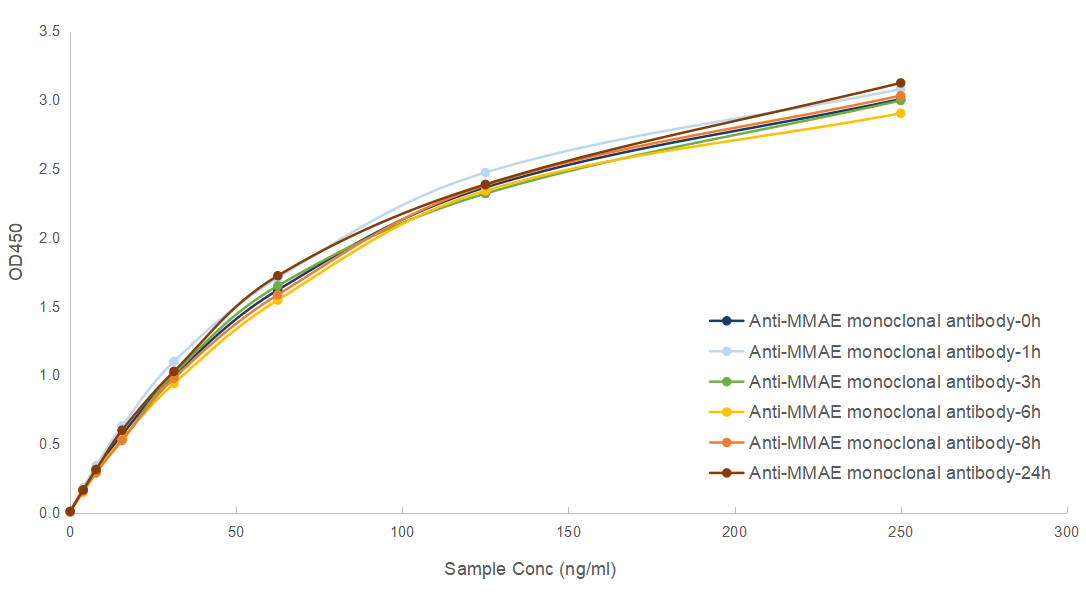
Anti-MMAE monoclonal antibody is stable at 37°C for 24 hours, equivalent to store at -80°C for 4.5 years without performance reduction. Welcome to contact us for more validation and testing data.
References
- Khongorzul P, Ling CJ, Khan FU, et al. (2019). Antibody-Drug Conjugates: A Comprehensive Review. Molecular Cancer Research. 18 (1): 3-19.
- Dean AQ, Luo S, Twomey JD, et al. (2021). Targeting cancer with antibody-drug conjugates: Promises and challenges. mAbs. 13(1), 1951427.
- Akaiwa M, Dugal-Tessier J, Mendelsohn BA. (2020). Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives. Chemical and Pharmaceutical Bulletin (Tokyo). 68(3):201-211.
| Cat_N | Product Name | |
| DAG-WT669 | MMAE [KLH] | Inquiry |
| DAG603S | MMAE [BSA] | Inquiry |
| DAG-WZ1001 | MMAE [HRP] | Inquiry |
| CABT-L3100 | Rabbit Anti-vc-PAB-MMAE polyclonal antibody | Inquiry |
| CABT-B8992 | Anti-MMAE monoclonal antibody, clone 3F3 | Inquiry |
| DEIABL312 | MMAE ADC EIA Kit | Inquiry |
| DEIABL314 | Intact MMAE ADC ELISA Kit | Inquiry |