Filter By Product Search for
AAV
 Loading...
Loading...
AAV Products
AAV Full Name
Adeno-associated virus
AAV Introduction
AAVs are effectively used to treat a number of genetic and acquired human diseases, more specifically, monogenic hereditary ones, i.e., diseases caused by mutations in one gene. Today, many AAV-based gene therapy medications are being investigated in preclinical and clinical trials. AAVs are small non-enveloped DNA viruses belonging to the Parvoviridae family, that were first isolated in 1965, as a contaminant in preparations of a simian adenovirus (Ad). The viruses were found incompetent to productively infect cells without a co-infection by a helper virus, usually an Ad or any type of herpesviruses, and thereby they were named as "adeno-associated", and classified into the Dependovirus genus.
Although AAVs have a high seroprevalence in humans (it has been estimated that 50% to 96% of the human population is seropositive for the second serotype of AAV (AAV2) depending on age and ethnic group), they, however, were not linked to any disease neither in humans, nor in any other species. Different AAVs have not only been detected in primates isolates, but also from avian, caprine, bovine, and equine stocks. Aside from AAV5 being the most divergent, all AAVs share a similar structure and properties. AAVs are easy to manipulate, as their particles can maintain biological stability in extreme conditions of pH and temperature. They share a genome of approximately 4.7 kb single-stranded DNA packed into an icosahedral, non-enveloped capsid with a diameter of 20–25 nm. The AAV genome consists mainly of two viral genes: rep (replication) and cap (capsid), flanked by inverted terminal repeats (ITRs).
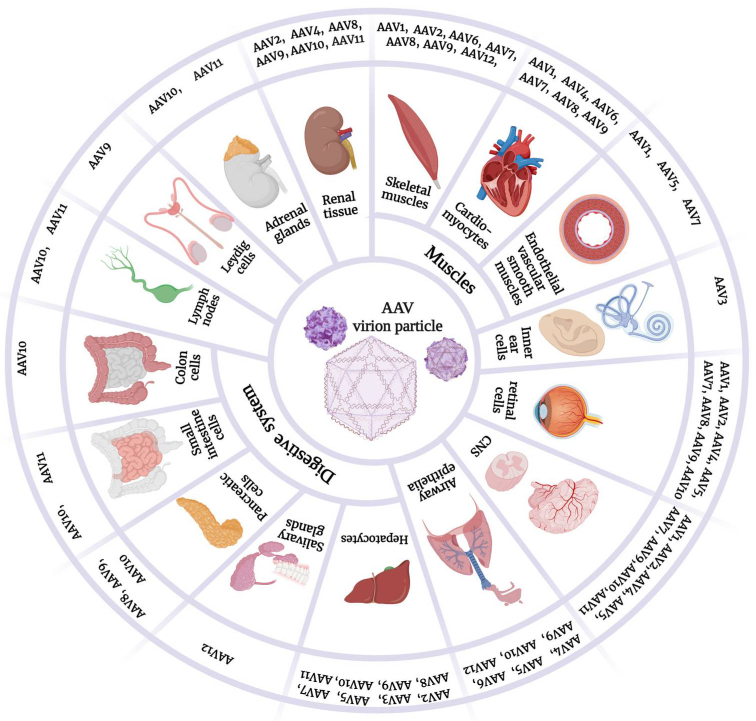 Figure 1. Variant tropisms of AAV serotypes.
Figure 1. Variant tropisms of AAV serotypes.
(Source: Cells. 2023)
Depending on their serotype, AAVs can have specific tropism for specific organs and tissues of the body. There are different AAV serotypes that vary in many aspects. Next, each serotype will be separately discussed. Table 1 summarizes the characteristics and properties of AAV serotypes, and Figure 1 demonstrates their variant tropisms.
Alternate Names for AAV
AAV
Adeno-associated virus
AAV-based in vivo gene therapy for neurological disorders
Recent advancements in gene supplementation therapy are expanding the options for the treatment of neurological disorders. Among the available delivery vehicles, adeno-associated virus (AAV) is often the favoured vector. However, the results have been variable, with some trials dramatically altering the course of disease whereas others have shown negligible efficacy or even unforeseen toxicity. Unlike traditional drug development with small molecules, therapeutic profiles of AAV gene therapies are dependent on both the AAV capsid and the therapeutic transgene.
Knowledge is growing about factors that impact the translation of preclinical studies to humans, including the administration route, timing of treatment, immune responses and limitations of available model systems. The field is also developing potential solutions to mitigate adverse effects, including AAV capsid engineering and designs to regulate transgene expression. At the same time, preclinical research is addressing new frontiers of gene supplementation for neurological disorders, with a focus on mitochondrial and neurodevelopmental disorders. Reseachers describe the current state of AAV-mediated neurological gene supplementation therapy, including critical factors for optimizing the safety and efficacy of treatments, as well as unmet needs in this field.
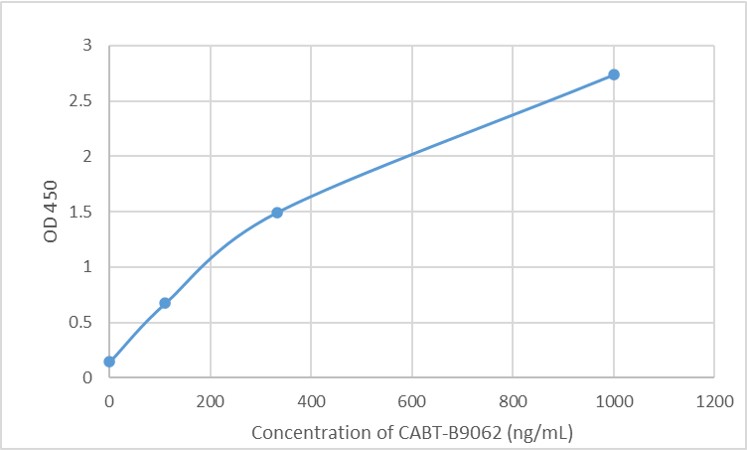
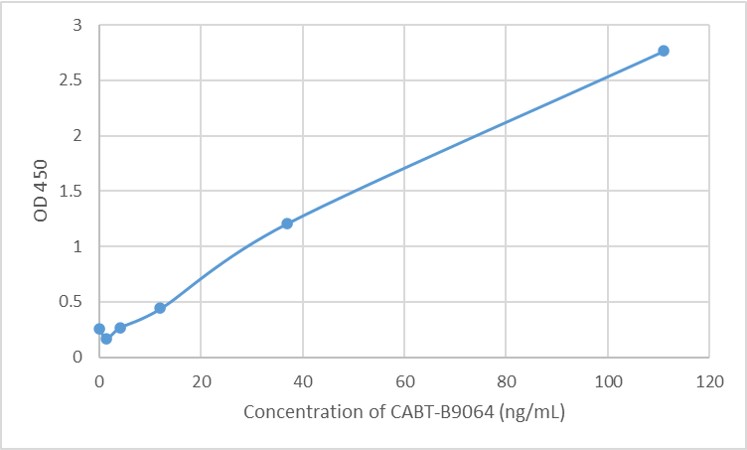
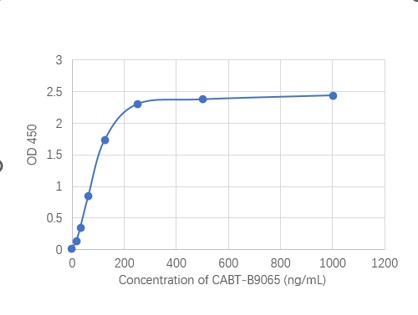
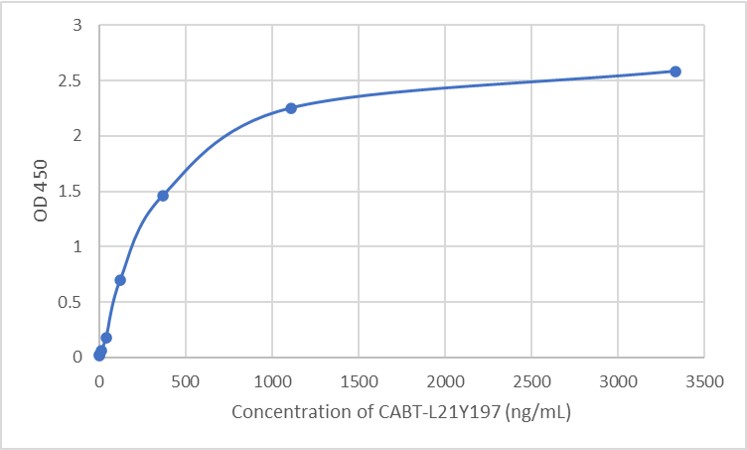
- AAV titration ELISA

- Anti-AAV Antibody ELISA Kits

- Humanized AAV Antibodies

- Mouse Anti-AAV8 ELISA Kit, Quantitative

- 1. Issa S.S., et al. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells. 2023;12:785.
2. Ling, Q., et al. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov 22. 2023, 789–806.