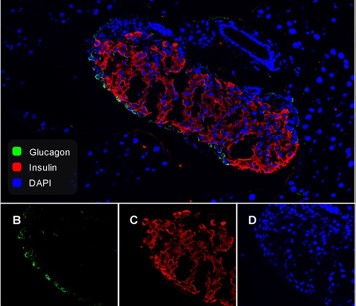
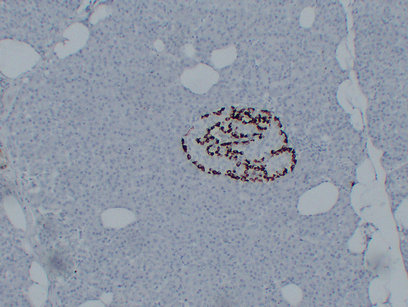
Anti-Human GLP-1(aa 7-17) monoclonal antibody
Mouse Anti-Human GLP-1(aa 7-17) monoclonal antibody for ELISA, IHC
Specifications
Antibody Isotype
IgG1, κ
Clone
N92496
Species Reactivity
Human, Mouse
Immunogen
Synthetic GLP-1(7-17)
Conjugate
Unconjugated
Applications
Application Notes
This clone binds free GLP-1(7-37) and free GLP-1(7-36)amide. Not recommended for use in sandwich ELISA
Target
Alternative Names
GCG; glucagon; GLP1; GLP2; GRPP; preproglucagon
Entrez Gene ID
UniProt ID
Custom Antibody Labeling
We offer labeled antibodies using our catalogue antibody products and a broad range of intensely fluorescent dyes and labels including HRP, biotin, ALP, Alexa Fluor® dyes, DyLight® Fluor dyes, R-phycoerythrin (R-PE), at scales from less than 100 μg up to 1 g of IgG antibody. Learn More
Citations
Have you cited DCABY-4434 in a publication? Let us know and earn a reward for your research.
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|---|---|---|---|---|---|
| Anti-Mouse IgG1 polyclonal antibody [HRP] | CPBT-68024GM | ELISA | Goat |
|
Inquiry | |
| Anti-Mouse IgG1 polyclonal antibody [R-PE] | CPBT-68086GM | FC | Goat |
|
Inquiry | |
| Anti-Mouse IgG1 polyclonal antibody [FITC] | CPBT-68023GM | ELISA FLISA ICC | Goat |
|
Inquiry | |
| Recombinant Alpaca Anti-Mouse IgG1 Fc monoclonal antibody, clone WII413 [Alexa Fluor®647] | CABT-L4221 | IF WB ELISA | Alpaca |
|
Inquiry | |
| Anti-Mouse IgG1 polyclonal antibody [AP] | CPBT-68022GM | IHC-Fr ELISA IHC-P WB | Goat |
|
Inquiry | |
| Anti-Mouse IgG1 monoclonal antibody, clone LO-MG1-2 [Biotin] | CABT-54626RM | ELISA | Rat |
|
Inquiry | |
| Anti-Mouse IgG1 monoclonal antibody, clone LO-MG1-2 [FITC] | CABT-54627RM | FC | Rat |
|
Inquiry | |
| Anti-Mouse IgG1 monoclonal antibody, clone LO-MG1-2 [HRP] | CABT-54628RM | ELISA | Rat |
|
Inquiry | |
| Recombinant Alpaca Anti-Mouse IgG1 Fc monoclonal antibody, clone WII413 | CABT-L4220 | IF WB ELISA | Alpaca |
|
Inquiry |
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
Paracrine regulation of somatostatin secretion by insulin and glucagon in mouse pancreatic islets
DIABETOLOGIA
Authors: Svendsen, Berit; Holst, Jens J.
Aims/hypothesis The endocrine pancreas comprises the islets of Langerhans, primarily consisting of beta cells, alpha cells and delta cells responsible for secretion of insulin, glucagon and somatostatin, respectively. A certain level of intra-islet communication is thought to exist, where the individual hormones may reach the other islet cells and regulate their secretion. Glucagon has been demonstrated to importantly regulate insulin secretion, while somatostatin powerfully inhibits both insulin and glucagon secretion. In this study we investigated how secretion of somatostatin is regulated by paracrine signalling from glucagon and insulin. Methods Somatostatin secretion was measured from perfused mouse pancreases isolated from wild-type as well as diphtheria toxin-induced alpha cell knockdown, and global glucagon receptor knockout (Gcgr(-/-)) mice. We studied the effects of varying glucose concentrations together with infusions of arginine, glucagon, insulin and somatostatin, as well as infusions of antagonists of insulin, somatostatin and glucagon-like peptide 1 (GLP-1) receptors. Results A tonic inhibitory role of somatostatin was demonstrated with infusion of somatostatin receptor antagonists, which significantly increased glucagon secretion at low and high glucose, whereas insulin secretion was only increased at high glucose levels. Infusion of glucagon dose-dependently increased somatostatin secretion approximately twofold in control mice. Exogenous glucagon had no effect on somatostatin secretion inGcgr(-/-)mice, and a reduced effect when combined with the GLP-1 receptor antagonist exendin 9-39. Diphtheria toxin-induced knockdown of glucagon producing cells led to reduced somatostatin secretion in response to 12 mmol/l glucose and arginine infusions. InGcgr(-/-)mice (where glucagon levels are dramatically increased) overall somatostatin secretion was increased. However, infusion of exendin 9-39 inGcgr(-/-)mice completely abolished somatostatin secretion in response to glucose and arginine. Neither insulin nor an insulin receptor antagonist (S961) had any effect on somatostatin secretion. Conclusions/interpretation Our findings demonstrate that somatostatin and glucagon secretion are linked in a reciprocal feedback cycle with somatostatin inhibiting glucagon secretion at low and high glucose levels, and glucagon stimulating somatostatin secretion via the glucagon and GLP-1 receptors.
Plasma dosage of ghrelin, IGF-1, GLP-1 and leptin related to gastric emptying and esophageal pH-impedance in children with obesity
JOURNAL OF ENDOCRINOLOGICAL INVESTIGATION
Authors: Quitadamo, P.; Zenzeri, L.; Mozzillo, E.; Giorgio, V.; Rocco, A.; Franzese, A.; Nardone, G.; Staiano, A.
Purpose The main aim of the study was to assess the relationship between leptin, ghrelin, insulin-like growth factor 1 (IGF-1), and glucagon-like peptide 1 (GLP-1) blood levels and gastric motility in children with obesity compared to healthy children. Secondary aims were to assess the possible association between these hormones and obesity, reflux impedance parameters, reflux symptoms, other GI disorders, and quality-of-life scores within the same groups. Methods Children with obesity plus GERD symptoms and 2 control groups of children with obesity without GERD and healthy lean children aged 4-17 years underwent an auxological evaluation, an assessment of gastro-intestinal symptoms and quality of life, hormonal dosages, and an evaluation of gastric emptying time (GET) through 13C-octanoic acid breath test. Results No significant association was found between hormones and gastric motility. Leptin and ghrelin levels were significantly associated with obesity parameters. No significant differences were found between GET and hormones of the patients with obesity, either with or without GERD. Conclusion Although we found an association between auxological parameters and both leptin and ghrelin levels, this association did not imply an effect on the upper GI motility. Therefore, our hypothesis that alterations of these hormones in children with obesity could affect gastric emptying, triggering GERD, was not supported by our data.
Online Inquiry
Catalog # DCABY-4434
Related Products
By targets
Related Resources
Ordering Information
Payment methods we support:
Invoice / Purchase Order
Credit card
Invoice / Purchase Order
Credit card
![]()