Metabolic profile of methylazoxymethanol model of schizophrenia in rats and effects of three antipsychotics in long-acting formulation
TOXICOLOGY AND APPLIED PHARMACOLOGY
Authors: Horska, Katerina; Kotolova, Hana; Karpisek, Michal; Babinska, Zuzana; Hammer, Tomas; Prochazka, Jiri; Stark, Tibor; Micale, Vincenzo; Ruda-Kucerova, Jana
Abstract
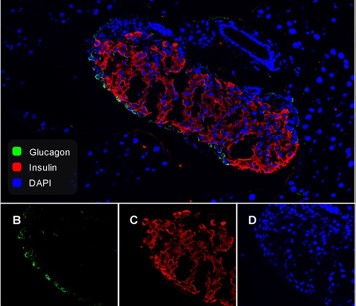
Mortality in psychiatric patients with severe mental illnesses reaches a 2-3 times higher mortality rate compared to the general population, primarily due to somatic comorbidities. A high prevalence of cardiovascular morbidity can be attributed to the adverse metabolic effects of atypical antipsychotics (atypical APs), but also to metabolic dysregulation present in drug-naive patients. The metabolic aspects of neurodevelopmental schizophrenia-like models are understudied. This study evaluated the metabolic phenotype of a methylazoxymethanol (MAM) schizophrenia-like model together with the metabolic effects of three APs [olanzapine (OLA), risperidone (RIS) and haloperidol (HAL)] administered via long-acting formulations for 8 weeks in female rats. Body weight, feed efficiency, serum lipid profile, gastrointestinal and adipose tissue-derived hormones (leptin, ghrelin, glucagon and glucagon-like peptide 1) were determined. The lipid profile was assessed in APs-naive MAM and control cohorts of both sexes. Body weight was not altered by the MAM model, though cumulative food intake and feed efficiency was lowered in the MAM compared to CTR animals. The effect of the APs was also present; body weight gain was increased by OLA and RIS, while OLA induced lower weight gain in the MAM rats. Further, the MAM model showed lower abdominal adiposity, while OLA increased it. Serum lipid profile revealed MAM model-induced alterations in both sexes; total, HDL and LDL cholesterol levels were increased. The MAM model did not exert significant alterations in hormonal parameters except for elevation in leptin level. The results support intrinsic metabolic dysregulation in the MAM model in both sexes, but the MAM model did not manifest higher sensitivity to metabolic effects induced by antipsychotic treatment.
Stabilizing Cellular Barriers: Raising the Shields Against COVID-19
FRONTIERS IN ENDOCRINOLOGY
Authors: Hanchard, Julia; Capo-Velez, Coral M.; Deusch, Kai; Lidington, Darcy; Bolz, Steffen-Sebastian
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its clinical manifestation (COVID-19; coronavirus disease 2019) have caused a worldwide health crisis. Disruption of epithelial and endothelial barriers is a key clinical turning point that differentiates patients who are likely to develop severe COVID-19 outcomes: it marks a significant escalation in respiratory symptoms, loss of viral containment and a progression toward multi-organ dysfunction. These barrier mechanisms are independently compromised by known COVID-19 risk factors, including diabetes, obesity and aging: thus, a synergism between these underlying conditions and SARS-CoV-2 mechanisms may explain why these risk factors correlate with more severe outcomes. This review examines the key cellular mechanisms that SARS-CoV-2 and its underlying risk factors utilize to disrupt barrier function. As an outlook, we propose that glucagon-like peptide 1 (GLP-1) may be a therapeutic intervention that can slow COVID-19 progression and improve clinical outcome following SARS-CoV-2 infection. GLP-1 signaling activates barrier-promoting processes that directly oppose the pro-inflammatory mechanisms commandeered by SARS-CoV-2 and its underlying risk factors.
![]()