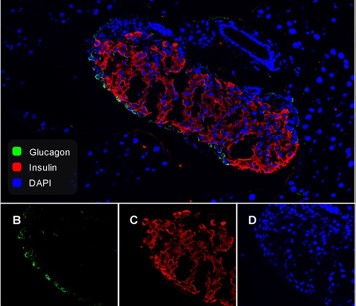
Ligand-Specific Factors Influencing GLP-1 Receptor Post-Endocytic Trafficking and Degradation in Pancreatic Beta Cells
INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES
Authors: Fang, Zijian; Chen, Shiqian; Manchanda, Yusman; Bitsi, Stavroula; Pickford, Philip; David, Alessia; Shchepinova, Maria M.; Correa, Ivan R., Jr.; Hodson, David J.; Broichhagen, Johannes; Tate, Edward W.; Reimann, Frank; Salem, Victoria; Rutter, Guy A.; Tan, Tricia; Bloom, Stephen R.; Tomas, Alejandra; Jones, Ben
Abstract
The glucagon-like peptide-1 receptor (GLP-1R) is an important regulator of blood glucose homeostasis. Ligand-specific differences in membrane trafficking of the GLP-1R influence its signalling properties and therapeutic potential in type 2 diabetes. Here, we have evaluated how different factors combine to control the post-endocytic trafficking of GLP-1R to recycling versus degradative pathways. Experiments were performed in primary islet cells, INS-1 832/3 clonal beta cells and HEK293 cells, using biorthogonal labelling of GLP-1R to determine its localisation and degradation after treatment with GLP-1, exendin-4 and several further GLP-1R agonist peptides. We also characterised the effect of a rare GLP1R coding variant, T149M, and the role of endosomal peptidase endothelin-converting enzyme-1 (ECE-1), in GLP1R trafficking. Our data reveal how treatment with GLP-1 versus exendin-4 is associated with preferential GLP-1R targeting towards a recycling pathway. GLP-1, but not exendin-4, is a substrate for ECE-1, and the resultant propensity to intra-endosomal degradation, in conjunction with differences in binding affinity, contributes to alterations in GLP-1R trafficking behaviours and degradation. The T149M GLP-1R variant shows reduced signalling and internalisation responses, which is likely to be due to disruption of the cytoplasmic region that couples to intracellular effectors. These observations provide insights into how ligand- and genotype-specific factors can influence GLP-1R trafficking.
Gastrointestinal adverse events with insulin glargine/lixisenatide fixed-ratio combination versus glucagon-like peptide-1 receptor agonistsin people with type 2 diabetes mellitus: A network meta-analysis
DIABETES OBESITY & METABOLISM
Authors: Rayner, Christopher K.; Wu, Tongzhi; Aroda, Vanita R.; Whittington, Craig; Kanters, Steve; Guyot, Patricia; Shaunik, Alka; Horowitz, Michael
Abstract
Aims Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are the recommended first injectable therapy in type 2 diabetes. However, long-term persistence is suboptimal and partly attributable to gastrointestinal tolerability, particularly during initiation/escalation. Gradual titration of fixed-ratio combination GLP-1 RA/insulin therapies may improve GLP-1 RA gastrointestinal tolerability. We compared gastrointestinal adverse event (AE) rates for iGlarLixi versus GLP-1 RAs during the first 12 weeks of therapy, including a sensitivity analysis with IDegLira. Materials and methods The PICO framework was used to identify studies from MEDLINE, EMBASE and CENTRAL searches using a proprietary, web-based, standardized tool with single data extraction. Gastrointestinal AEs were modelled using a Bayesian network meta-analysis (NMA), using fixed and random effects for each recommended dose (treatment-specific NMA) and class (drug-class NMA). Results Treatment-specific NMA included 17 trials (n = 9030; 3665 event-weeks). Nausea rates were significantly lower with iGlarLixi versus exenatide 10 mu g twice daily (rate ratio: 0.32; 95% credible interval: 0.15, 0.66), once-daily lixisenatide 20 mu g (0.35; 0.24, 0.50) and liraglutide 1.8 mg once daily (0.48; 0.23, 0.98). Rates were numerically, but not statistically, lower versus once-weekly semaglutide 1 mg (0.60; 0.30, 1.23) and dulaglutide 1.5 mg (0.60; 0.29, 1.26), and numerically, but not statistically, higher versus once-weekly exenatide (1.91; 0.91, 4.03). Sensitivity analysis results were similar. In a naive, pooled analysis, vomiting was lower with iGlarLixi versus other GLP-1 RAs. Conclusions During the first 12 weeks of treatment, iGlarLixi was generally associated with less nausea and vomiting than single-agent GLP-1 RAs. Enhanced gastrointestinal tolerability with fixed-ratio combinations may favour treatment persistence.
![]()