Up-Regulation of leucocytes Genes Implicated in Telomere Dysfunction and Cellular Senescence Correlates with Depression and Anxiety Severity Scores
PLOS ONE
Authors: Teyssier, Jean-Raymond; Chauvet-Gelinier, Jean-Christophe; Ragot, Sylviane; Bonin, Bernard
Abstract
Background: Major depressive disorder (MDD) is frequently associated with chronic medical illness responsible of increased disability and mortality. Inflammation and oxidative stress are considered to be the major mediators of the allostatic load, and has been shown to correlate with telomere erosion in the leucocytes of MDD patients, leading to the model of accelerated aging. However, the significance of telomere length as an exclusive biomarker of aging has been questioned on both methodological and biological grounds. Furthermore, telomeres significantly shorten only in patients with long lasting MDD. Sensitive and dynamic functional biomarkers of aging would be clinically useful to evaluate the somatic impact of MDD. Methodology: To address this issue we have measured in the blood leucocytes of MDD patients (N = 17) and controls (N = 16) the expression of two genes identified as robust biomarkers of human aging and telomere dysfunction: p16(INK4a) and STMN1. We have also quantified the transcripts of genes involved in the repair of oxidative DNA damage at telomeres (OGG1), telomere regulation and elongation (TERT), and in the response to biopsychological stress (FOS and DUSP1). Results: The OGG1, p16(INK4a), and STMN1 gene were significantly up-regulated (25 to 100%) in the leucocytes of MDD patients. Expression of p16(INK4a) and STMN1 was directly correlated with anxiety scores in the depression group, and that of p16(INK4a), STMN and TERT with the depression and anxiety scores in the combined sample (MDD plus controls). Furthermore, we identified a unique correlative pattern of gene expression in the leucocytes of MDD subjects. Conclusions: Expression of p16(INK4) and STMN1 is a promising biomarker for future epidemiological assessment of the somatic impact of depressive and anxious symptoms, at both clinical and subclinical level in both depressive patients and general population.
Distinctive roles of syntaxin binding protein 4 and its action target, TP63, in lung squamous cell carcinoma: a theranostic study for the precision medicine
BMC CANCER
Authors: Bilguun, Erkhem-Ochir; Kaira, Kyoichi; Kawabata-Iwakawa, Reika; Rokudai, Susumu; Shimizu, Kimihiro; Yokobori, Takehiko; Oyama, Tetsunari; Shirabe, Ken; Nishiyama, Masahiko
Abstract
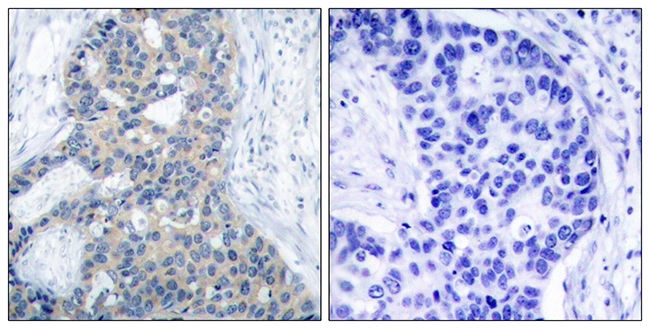
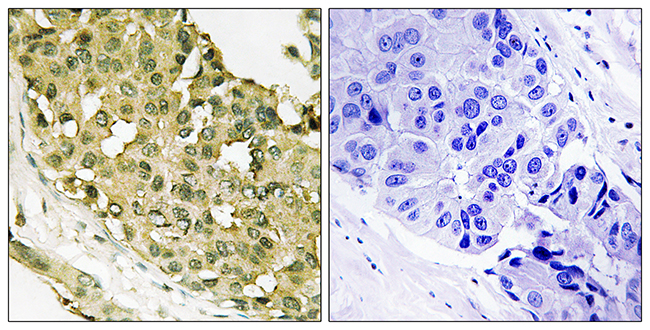
BackgroundLung squamous cell carcinoma (LSCC) remains a challenging disease to treat, and further improvements in prognosis are dependent upon the identification of LSCC-specific therapeutic biomarkers and/or targets. We previously found that Syntaxin Binding Protein 4 (STXBP4) plays a crucial role in lesion growth and, therefore, clinical outcomes in LSCC patients through regulation of tumor protein p63 (TP63) ubiquitination.MethodsTo clarify the impact of STXBP4 and TP63 for LSCC therapeutics, we assessed relevance of these proteins to outcome of 144 LSCC patients and examined whether its action pathway is distinct from those of currently used drugs in in vitro experiments including RNA-seq analysis through comparison with the other putative exploratory targets and/or markers.ResultsKaplan-Meier analysis revealed that, along with vascular endothelial growth factor receptor 2 (VEGFR2), STXBP4 expression signified a worse prognosis in LSCC patients, both in terms of overall survival (OS, p=0.002) and disease-free survival (DFS, p=0.041). These prognostic impacts of STXBP4 were confirmed in univariate Cox regression analysis, but not in the multivariate analysis. Whereas, TP63 (Delta Np63) closely related to OS (p=0.013), and shown to be an independent prognostic factor for poor OS in the multivariate analysis (p=0.0324). The action pathway of STXBP4 on suppression of TP63 (Delta Np63) was unique: Ingenuity pathway analysis using the knowledge database and our RNA-seq analysis in human LSCC cell lines indicated that 35 pathways were activated or inactivated in association with STXBP4, but the action pathway of STXBP4 was distinct from those of other current drug targets: STXBP4, TP63 and KDR (VEGFR2 gene) formed a cluster independent from other target genes of tumor protein p53 (TP53), tubulin beta 3 (TUBB3), stathmin 1 (STMN1) and cluster of differentiation 274 (CD274: programmed cell death 1 ligand 1, PD-L1). STXBP4 itself appeared not to be a potent predictive marker of individual drug response, but we found that TP63, main action target of STXBP4, might be involved in drug resistance mechanisms of LSCC.ConclusionSTXBP4 and the action target, TP63, could afford a key to the development of precision medicine for LSCC patients.
![]()