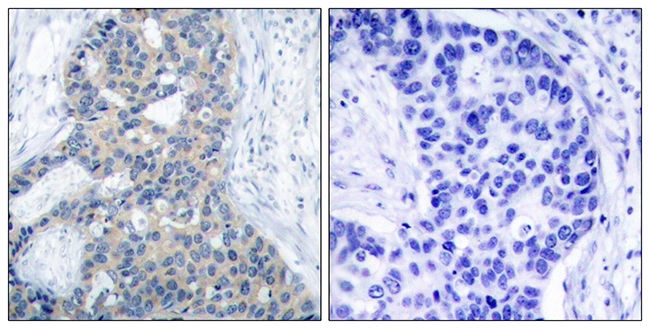
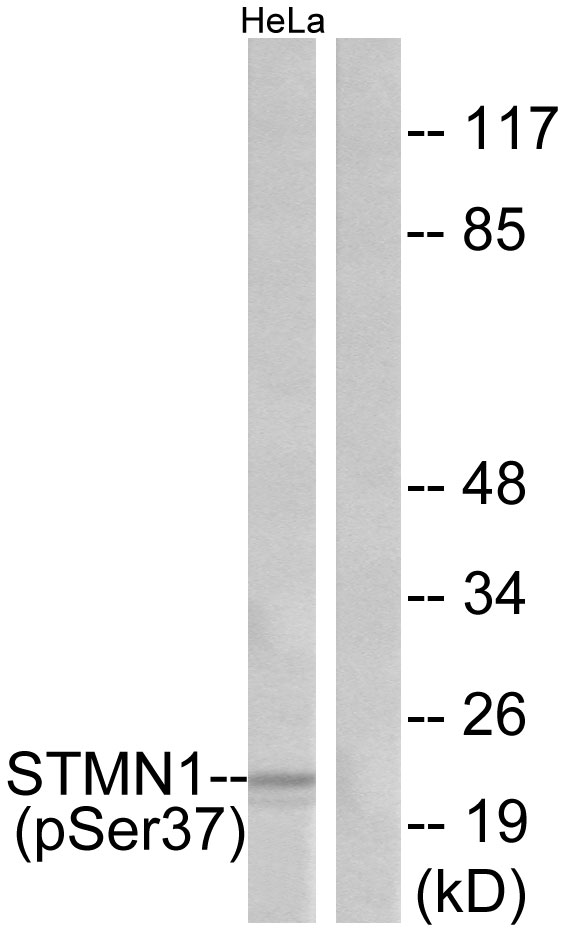
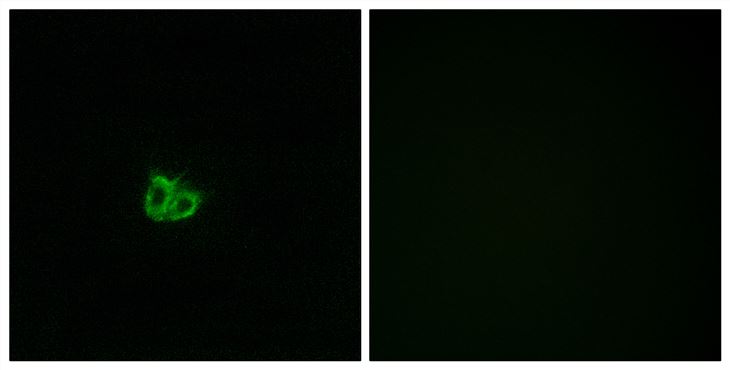
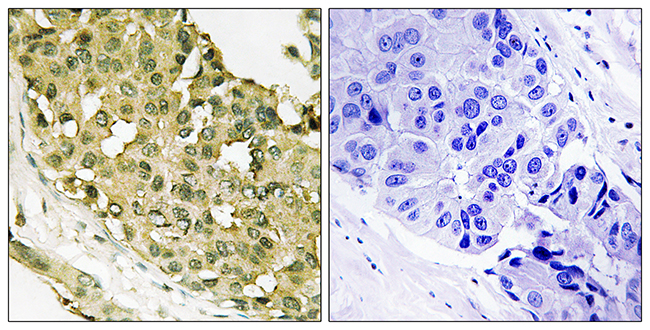
Stathmin genotype is associated with reexperiencing symptoms of posttraumatic stress disorder in Chinese earthquake survivors
PROGRESS IN NEURO-PSYCHOPHARMACOLOGY & BIOLOGICAL PSYCHIATRY
Authors: Cao, Chengqi; Wang, Li; Wang, Richu; Dong, Chongya; Qing, Yulan; Zhang, Xiangyang; Zhang, Jianxin
Abstract
Stathmin (STMN1) has been demonstrated as a regulator of fear processing across species, which implicates that it may be important in the etiopathogenesis of fear-related psychiatric disorders such as posttraumatic stress disorder (PTSD). This study examined the association between STMN1 rs182455 genotype, a single nucleotide polymorphism (SNP) located within or close to the putative transcriptional control region of STMN1 gene, and PTSD symptoms. A total of 326 Chinese adults who suffered from a deadly 2008 Wenchuan earthquake and unexpectedly lost their children during the disaster participated in this study. PTSD symptoms were measured with the PTSD Checklist (PCL). The Sequenom iPlex chemistries and the MassARRAY system were used to genotype the STMN1 rs182455 SNP. Our results indicated that the STMN1rs182455 genotype was not associated with severity of total PTSD symptoms in either females or males; however, it could significantly predict severity of PTSD's reexperiencing symptoms in females. The findings provide preliminary evidence supporting the important role of STMN1 in the development of PTSD, and expand extant knowledge on the genetic underpinnings of PTSD and the sex-specific expression of PTSD's symptoms. (C) 2013 Elsevier Inc. All rights reserved.
From mice to humans: Identification of commonly deregulated genes in mammary cancer via comparative SAGE studies
CANCER RESEARCH
Authors: Hu, YH; Sun, HX; Drake, J; Kittrell, F; Abba, MC; Deng, L; Gaddis, S; Sahin, A; Baggerly, K; Medina, D; Aldaz, CM
Abstract
Genetically engineered mouse mammary cancer models have been used over the years as systems to study human breast cancer. However, much controversy exists on the utility of such models as valid equivalents to the human cancer condition. To perform an interspecies gene expression comparative study in breast cancer we used a mouse model that most closely resembles human breast carcinogenesis. This system relies on the transplant of p53 null mouse mammary epithelial cells into the cleared mammary fat pads of syngeneic hosts. Serial analysis of gene expression (SAGE) was used to obtain gene expression profiles of normal and tumor samples from this mouse mammary cancer model (>300,000 mouse mammary-specific tags). The resulting mouse data were compared with 25 of our human breast cancer SAGE libraries (>2.5 million human breast-specific tags). We observed significant similarities in the deregulation of specific genes and gene families when comparing mouse with human breast cancer SAGE data. A total of 72 transcripts were identified as commonly deregulated in both species. We observed a systematic and significant down-regulation in all of the tumors from both species of various cytokines, including CXCL1 (GRO1), LIF, interleukin 6, and CCL2. All of the mouse and most human mammary tumors also displayed decreased expression of genes known to inhibit cell proliferation, including NFKBIA (IKBalpha), GADD45B, and CDKN1A (p21); transcription-related genes such as CEBP, JUN, JUNB, and ELF1; and apoptosis-related transcripts such as IER3 and GADD34/PPP1R15A. Examples of overexpressed transcripts in tumors from both species include proliferation-related genes such as CCND1, CKS1B, and STMN1 (oncoprotein 18); and genes related to other functions such as SEPW1, SDFR1, DNCI2, and SP110. Importantly, abnormal expression of several of these genes has not been associated previously with breast cancer, The consistency of these observations was validated in independent mouse and human mammary cancer sets. This is the first interspecies comparison of mammary cancer gene expression profiles. The comparative analysis of mouse and human SAGE mammary cancer data validates this p53 null mouse tumor model as a useful system closely resembling human breast cancer development and progression. More importantly, these studies are allowing us to identify relevant biomarkers of potential use in human studies while leading to a better understanding of specific mechanisms of human breast carcinogenesis.
![]()