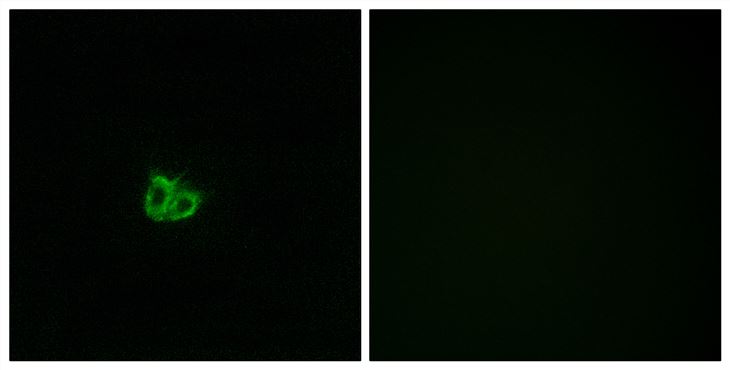
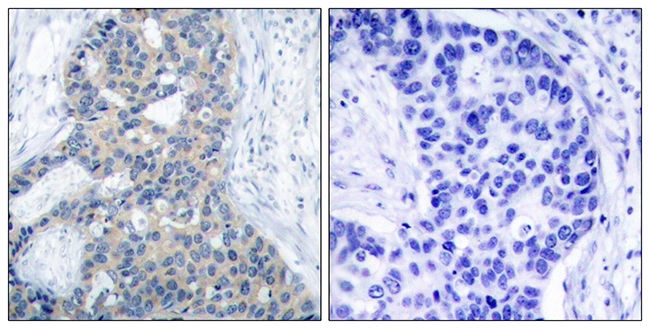
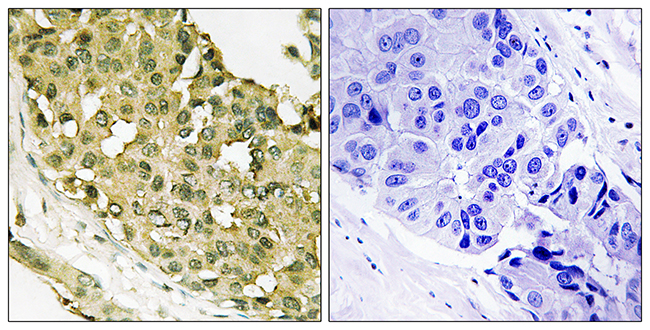
MicroRNA-101 regulated transcriptional modulator SUB1 plays a role in prostate cancer
ONCOGENE
Authors: Chakravarthi, B. V. S. K.; Goswami, M. T.; Pathi, S. S.; Robinson, A. D.; Cieslik, M.; Chandrashekar, D. S.; Agarwal, S.; Siddiqui, J.; Daignault, S.; Carskadon, S. L.; Jing, X.; Chinnaiyan, A. M.; Kunju, L. P.; Palanisamy, N.; Varambally, S.
Abstract
MicroRNA-101, a tumor suppressor microRNA (miR), is often downregulated in cancer and is known to target multiple oncogenes. Some of the genes that are negatively regulated by miR-101 expression include histone methyltransferase EZH2 (enhancer of zeste homolog 2), COX2 (cyclooxygenase-2), POMP (proteasome maturation protein), CERS6, STMN1, MCL-1 and ROCK2, among others. In the present study, we show that miR-101 targets transcriptional coactivator SUB1 homolog (Saccharomyces cerevisiae)/PC4 (positive cofactor 4) and regulates its expression. SUB1 is known to have diverse role in vital cell processes such as DNA replication, repair and heterochromatinization. SUB1 is known to modulate transcription and acts as a mediator between the upstream activators and general transcription machinery. Expression profiling in several cancers revealed SUB1 overexpression, suggesting a potential role in tumorigenesis. However, detailed regulation and function of SUB1 has not been elucidated. In this study, we show elevated expression of SUB1 in aggressive prostate cancer. Knockdown of SUB1 in prostate cancer cells resulted in reduced cell proliferation, invasion and migration in vitro, and tumor growth and metastasis in vivo. Gene expression analyses coupled with chromatin immunoprecipitation revealed that SUB1 binds to the promoter regions of several oncogenes such as PLK1 (Polo-like kinase 1), C-MYC, serine-threonine kinase BUB1B and regulates their expression. Additionally, we observed SUB1 downregulated CDKN1B expression. PLK1 knockdown or use of PLK1 inhibitor can mitigate oncogenic function of SUB1 in benign prostate cancer cells. Thus, our study suggests that miR-101 loss results in increased SUB1 expression and subsequent activation of known oncogenes driving prostate cancer progression and metastasis. This study therefore demonstrates functional role of SUB1 in prostate cancer, and identifies its regulation and potential downstream therapeutic targets of SUB1 in prostate cancer.
Stathmin1 overexpression in hypopharyngeal squamous cell carcinoma: A new promoter in FaDu cell proliferation and migration
INTERNATIONAL JOURNAL OF ONCOLOGY
Authors: Chen, Yan; Zhang, Qicheng; Ding, Chuanjin; Zhang, Xiaobo; Qiu, Xiaoxia; Zhang, Zhenxin
Abstract
Stathmin1, a microtubule-destabilizing phosphoprotein, is considered to play a crucial role in regulating cellular microtubule dynamics and controlling mitosis. Previous studies have showed that STMN1 is highly expressed in many human malignancies and is related to development, invasion and metastasis of tumors. However, its expression pattern, clinical performance and functional roles in hypopharyngeal squamous cell carcinoma (HSCC) have not been addressed. In this study, we found that STMN1 was significantly elevated in HSCC and its expression level was correlated with poor differentiation (P<0.001), clinical stage (P<0.001), large tumor size (P=0.001) and lymph node metastasis (P=0.008). A positive correlation between STMN1 and Ki-67 expression was also exhibited. High STMN1 expression predicted poor survival. Furthermore, we found that knockdown of STMN1 by siRNAs inhibited the FaDu cell proliferation and migration. Moreover, decreased STMN1 expression in FaDu cells reversed the acquisition of EMT phenotype by upregulating E-cadherin, as well as reduced vimentin expression at protein and mRNA levels. These results suggested that STMN1 plays an important role in proliferation and migration of HSCC and may be used as a potential prognostic biomarker or therapeutic target of HSCC.
![]()