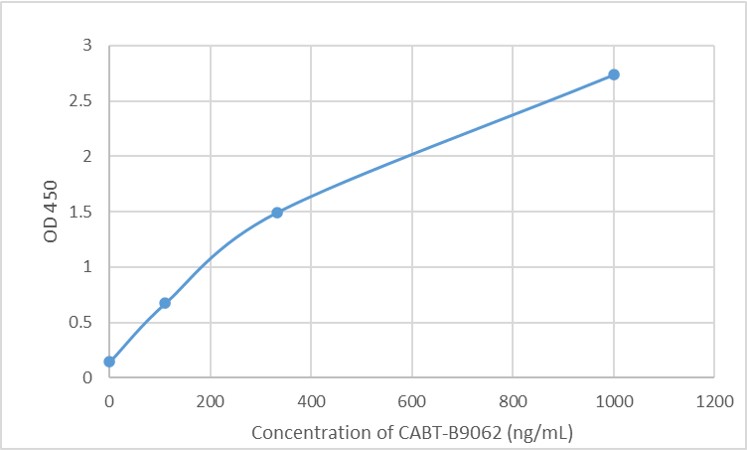
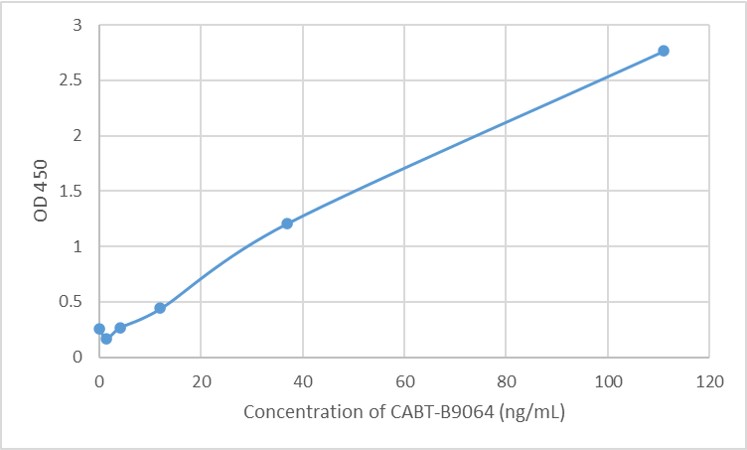
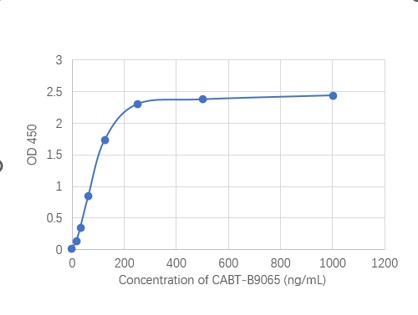
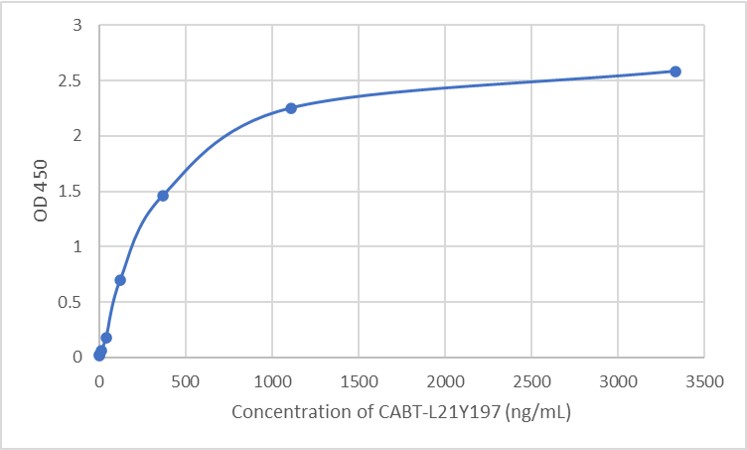
Targeting RyR2 with a phosphorylation site-specific nanobody reverses dysfunction of failing cardiomyocytes in rats
FASEB JOURNAL
Authors: Li, Tian; Shen, Yafeng; Lin, Fangxing; Fu, Wenyan; Liu, Shuowu; Wang, Chuqi; Liang, Jizhou; Fan, Xiaoyan; Ye, Xuting; Tang, Ying; Ding, Min; Yang, Yongji; Lei, Changhai; Hu, Shi
Abstract
Chronic PKA phosphorylation of ryanodine receptor 2 (RyR2) has been shown to increase diastolic sarcoplasmic reticulum (SR) Ca2+ leakage and lead to cardiac dysfunction. We hypothesize that intracellular gene delivery of an RyR2-targeting phosphorylation site-specific nanobody could preserve the contractility of the failing myocardium. In the present study, we acquired RyR2-specific nanobodies from a phage display library that were variable domains of Camelidae heavy chain-only antibodies. One of the nanobodies, AR185, inhibited RyR2 phosphorylation in vitro and was chosen for further investigation. We investigated the potential of adeno-associated virus (AAV)9-mediated cardiac expression of AR185 to combat postischemic heart failure (HF). AAV gene delivery elevated the intracellular expression of the AR185 protein in a rat model of ischemic HF, and this treatment normalized the systolic and diastolic dysfunction of the failing myocardium in vivo by reversing myocardial Ca2+ handling. Furthermore, AR185 gene transfer to failing cardiomyocytes reduced the frequency of SR calcium leaks, thereby restoring the attenuated intracellular calcium transients and SR calcium load. Moreover, AR185 gene transfer inhibited the PKA-mediated phosphorylation of RyR2 in failing cardiomyocytes. Our results provide preclinical experimental evidence that the cardiac expression of RyR2 nanobodies with AAV9 vectors is a promising therapeutic strategy for HF.-Li, T., Shen, Y., Lin, F., Fu, W., Liu, S., Wang, C., Liang, J., Fan, X., Ye, X., Tang, Y., Ding, M., Yang, Y., Lei, C., Hu, S. Targeting RyR2 with a phosphorylation site-specific nanobody reverses dysfunction of failing cardiomyocytes in rats.
Neuraminidase-mediated desialylation augments AAV9-mediated gene expression in skeletal muscle
JOURNAL OF GENE MEDICINE
Authors: Zhu, Hongling; Wang, Tao; Lye, Robert John; French, Brent A.; Annex, Brian H.
Abstract
BackgroundFollowing systemic delivery, AAV9-mediated gene expression is significantly increased in ischemic versus non-ischemic muscle, suggesting that AAV9 is an attractive vector for treating peripheral arterial disease. Potential mechanisms underlying ischemia-augmented expression include: (i) increased vascular permeability and (ii) unmasking of endogenous AAV9 receptors. In the present study, we aimed to reconstitute the ischemic induction of AAV9 in vivo, using local injection of histamine (to increase vascular permeability) and neuraminidase (to desialylate cell surface glycans). MethodsBioassays were performed to optimize the effects of histamine and neuraminidase after intramuscular injection. Histamine and/or neuraminidase were then injected intramuscularly shortly before intravenous injection of an AAV9 vector expressing luciferase. Luciferase expression was serially assessed with bioluminescence imaging. At the end of the study, tissues were harvested for assays of luciferase activity and AAV9 genome copy number aiming to assess AAV-mediated gene expression and transduction, respectively. ResultsIntramuscular injection of either neuraminidase or neuraminidase plus histamine significantly increased both transduction and gene expression, whereas histamine alone had little effect. Pre-injection with neuraminidase increased AAV9-mediated gene delivery by four- to nine-fold and luciferase activity by 60-100-fold. Luciferase activity in neuraminidase-injected muscle was >100-fold higher than in any off-target tissue (including heart, liver and brain). ConclusionsThe ischemic induction of AAV9-mediated gene expression in muscle can largely be reconstituted by pre-injecting neuraminidase intranmuscularly. This strategy may prove useful in future human gene therapy protocols as a quick and efficient means to selectively target systemically injected AAV9 to localized regions of muscle, thus decreasing the potential for adverse effects in off-target tissues.
![]()