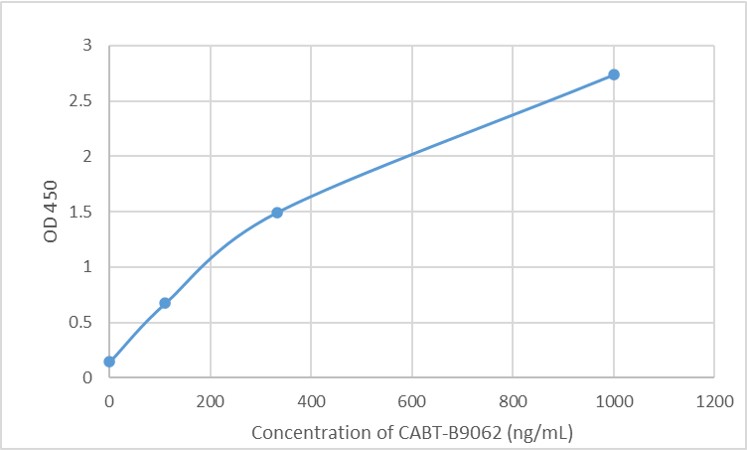
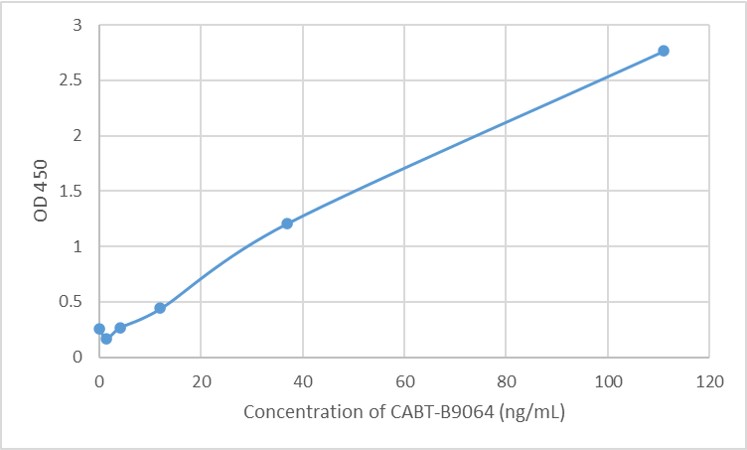
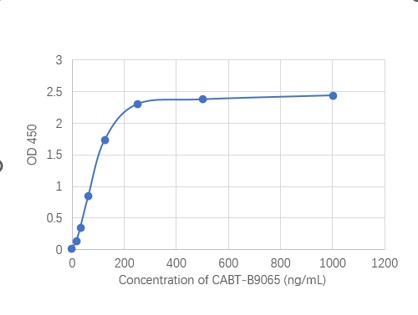
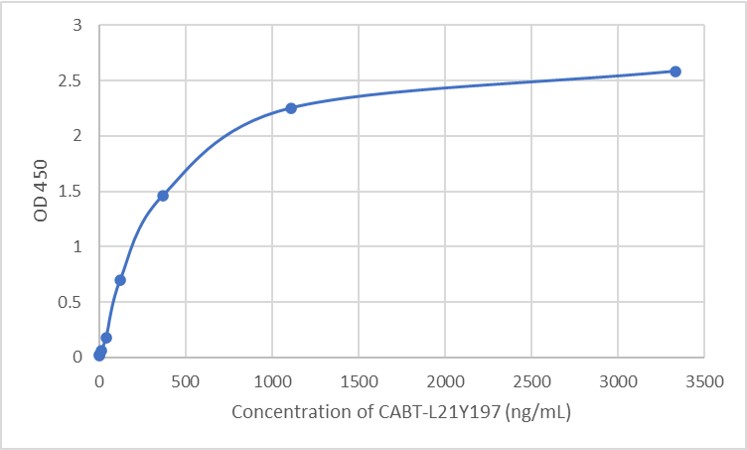
GTCDxᵀᴹ Anti-AAV9 antibody ELISA Kit
Regulatory status: For research use only, not for use in diagnostic procedures.
| No. | Components | Size | Storage Conditions |
| 1 | AAV9 Capsid Coated Microtiter Plate | 12 × 8 wells | 2-8°C |
| 2 | Negative Control, ready to use | 1 × 1 ml | 2-8°C |
| 3 | Anti-AAV9 Positive Control, ready to use | 1 × 1 ml | 2-8°C |
| 4 | Cut-off Control, 2 × concentrate | 1 × 0.5 ml | 2-8°C |
| 5 | Anti-human IgG Conjugate, ready to use | 1 × 12 ml | 2-8°C |
| 6 | Sample Diluent, ready to use | 1 × 50 ml | 2-8°C |
| 7 | Wash Buffer, 20 × concentrate | 1 × 50 ml | 2-8°C |
| 8 | TMB Substrate, ready to use | 2 × 6 ml | 2-8°C |
| 9 | Stop Solution, ready to use | 1 × 7 ml | 2-8°C |
| 10 | Instruction Manual | 1 |
2. All reagents and wash buffer should be used within 12 months from manufacturing date.
3. Before using, bring all components to room temperature (18-25°C). Upon assay completion ensure all components of the kit are returned to appropriate storage conditions.
4. The Substrate is light-sensitive and should be protected from direct sunlight or UV sources.
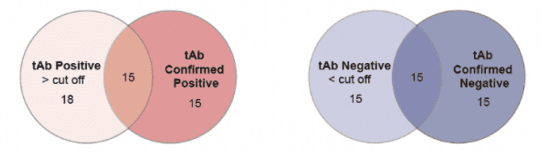
Non-scaled Venn diagram depicting the relationship between total (tAb) and specific confirmed total antibodies in samples. The results are highly consistent, indicating that ELISA has high accuracy in detecting AAV antibodies.
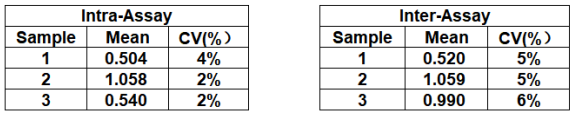
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
Adeno-associated virus (AAV), which belongs to the parvovirus family, is the simplest single-stranded DNA virus without envelope found so far. AAV has become one of the most important gene carriers in the field of gene therapy because it cannot self-replicate, does not cause any human diseases, long-lasting gene expression and has high tissue specificity, providing a new possibility for the treatment of a variety of genetic diseases. Currently, there are 13 serotypes of AAV based on capsid protein composition (AAV1-AAV13), which differ in their proclivity and transduction efficiency in different tissues, of which AAV9 was found to have the highest proclivity to the central nervous system.
AAV9, which can bypass the blood-brain barrier and enter the central nervous system without the need to use other drugs or physical assistance, exhibits high transgenic expression and extensive central nervous system transduction in neonatal and adult mice, and is the most widely studied intravenous delivery vector. AAV9 is typically used to repair defective genes, such as lysosomal storage disease and spinal muscular atrophy type 1; to suppress dominant genetic mutations such as Huntington's disease; or to introduce a disease-modifying gene that may alleviate neuropathology, as in the case of Alzheimer's disease. In addition, due to the high efficiency of AAV9 transduction of spinal cord MN, many preclinical studies have focused on motor neuron diseases, especially spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).
While AAV vectors are characteristically low in immunogenicity, the body's immune system can still recognize and respond to them, about 30%-80% of people have pre-existing anti-AAV antibodies, which have the possibility of affecting the effectiveness of gene therapy. Therefore, the detection of immune response to AAV vector in AAV9 therapy is very important to evaluate the safety and success rate of gene therapy. Creative Diagnostics has developed an ELISA kit for the detection of AAV9 antibodies, which can efficiently detect circulating AAV9 antibodies in human serum and ensure the efficacy and safety of gene therapy as much as possible.
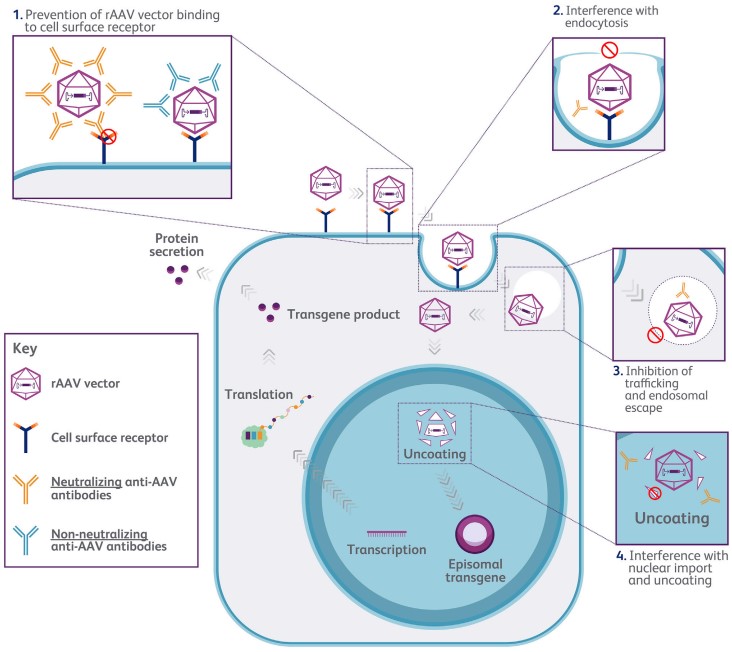 Figure 1. schematic diagram of anti-AAV neutralizing antibody inhibiting transgenic expression
Figure 1. schematic diagram of anti-AAV neutralizing antibody inhibiting transgenic expression
(Source: Schulz M, et al. 2023)
Adeno-associated virus 9 antibody ELISA Kit
Adeno-associated virus type 9 ELISA Kit
Anti-AAV9 antibody ELISA
References
1. Saraiva J, et al. Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9. J Control Release. 2016 Nov 10;241:94-109.
2. Schulz M, et al. Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol Ther. 2023 Mar 1;31(3):616-630.
Q: I would need to know the specific antibodies that do the detection. Is this available?
A: Human anti-AAV9 Capsids IgG.
Q: Do we have to pre-dilute our samples?
A: Dilution: 1:50
Q: Are the negative / positive / cut-off controls serum, plasma or buffer?
A: All three of our controls contained antibody buffers and no serum components
Q: How did you determine the reference limit? Was it according to CLSI EP28-A3c guidelines?
A: Our method is similar to CLSI EP28-A3c guidelines, both of which use statistical methods. But we don't refer to this file
Q: How many samples did you use?
A: AAV9(118 samples)
Q: Which samples (sera, plasma, etc.) did you use to determine the cut-off?
A: Serum
Q: How did you determine the cut-off point?
A: We used our kit and a confirmed assay method in the literature to determine the cut-off
Intrathecal AAV9/AP4M1 gene therapy for hereditary spastic paraplegia 50 shows safety and efficacy in preclinical studies
J Clin Invest
Authors: Chen X, Dong T, Hu Y, De Pace R, Mattera R, Eberhardt K, Ziegler M, Pirovolakis T, Sahin M, Bonifacino JS, Ebrahimi-Fakhari D, Gray SJ.
Endothelial Retargeting of AAV9 In Vivo
Adv Sci (Weinh)
Authors: Bozoglu T, Lee S, Ziegler T, Jurisch V, Maas S, Baehr A, Hinkel R, Hoenig A, Hariharan A, Kim CI, Decker S, Sami H, Koppara T, Oellinger R, Müller OJ, Frank D, Megens R, Nelson P, Weber C, Schnieke A, Sperandio M, Santamaria G, Rad R, Moretti A, Laugwitz KL, Soehnlein O, Ogris M, Kupatt C.
- AAV For Gene Therapy
- AAV9 Gene Therapy
- Gene Therapy
- GTCDxᵀᴹ Anti-AAV Antibody ELISA Kits
 Anti-AAV Antibody ELISA Kits
Anti-AAV Antibody ELISA Kits
Invoice / Purchase Order
Credit card
![]()