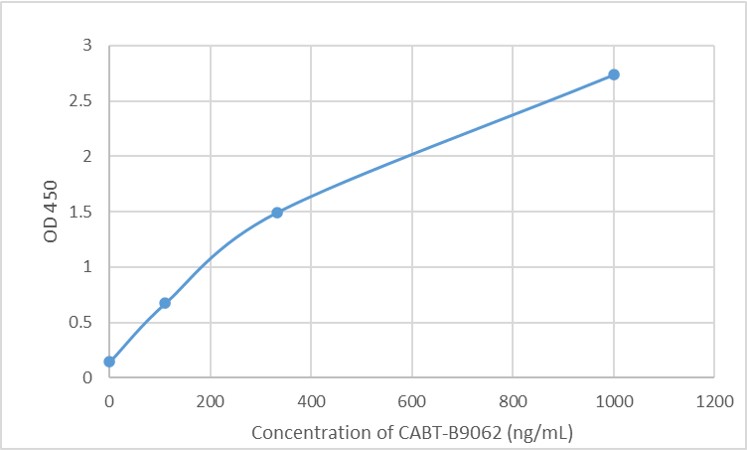
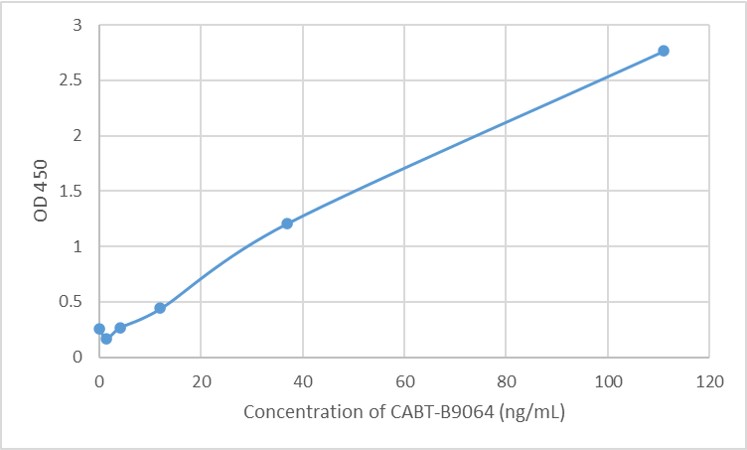
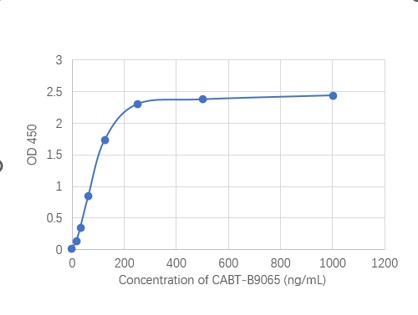
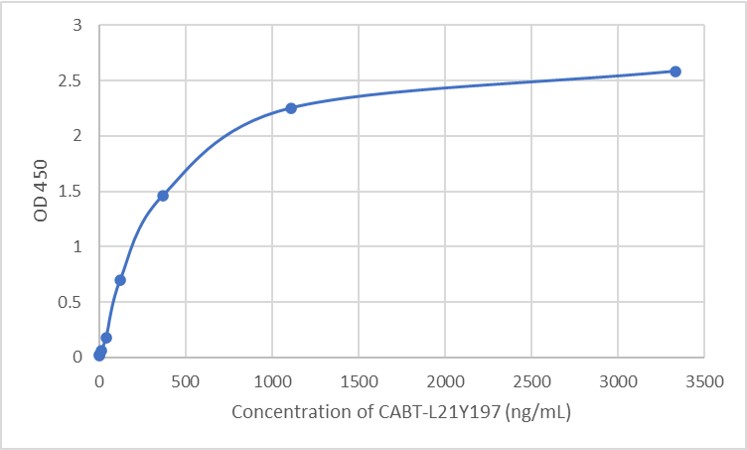
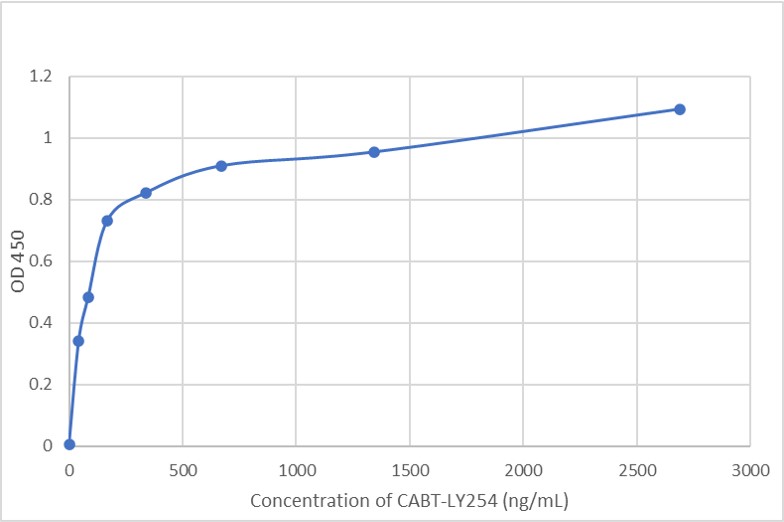
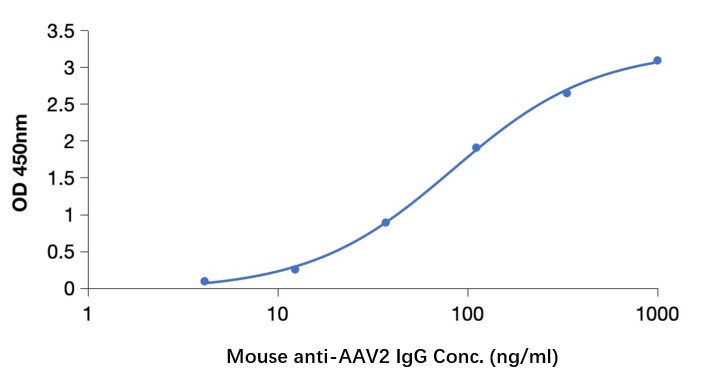
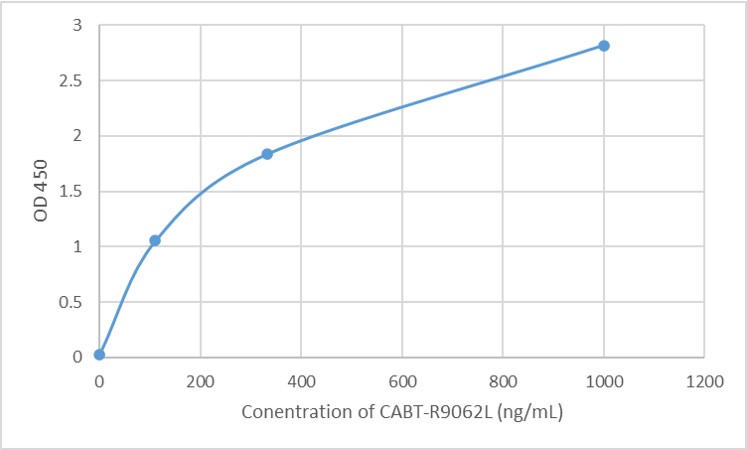
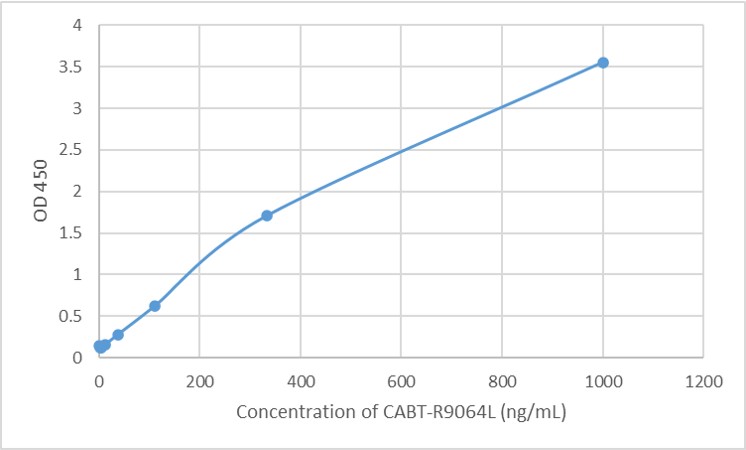
GTCDxᵀᴹ Anti-AAV8 antibody ELISA Kit
Regulatory status: For research use only, not for use in diagnostic procedures.
| No. | Components | Size | Storage Conditions |
| 1 | AAV8 Capsid Coated Microtiter Plate | 12 × 8 wells | 2-8°C |
| 2 | Negative Control, ready to use | 1 × 1 ml | 2-8°C |
| 3 | Anti-AAV8 Positive Control, ready to use | 1 × 1 ml | 2-8°C |
| 4 | Cut-off Control, ready to use | 1 × 1 ml | 2-8°C |
| 5 | Anti-human IgG Conjugate, ready to use | 1 × 12 ml | 2-8°C |
| 6 | Sample Diluent, ready to use | 1 × 50 ml | 2-8°C |
| 7 | Wash Buffer, 20 × concentrate | 1 × 50 ml | 2-8°C |
| 8 | TMB Substrate, ready to use | 2 × 6 ml | 2-8°C |
| 9 | Stop Solution, ready to use | 1 × 7 ml | 2-8°C |
| 10 | Instruction Manual | 1 |
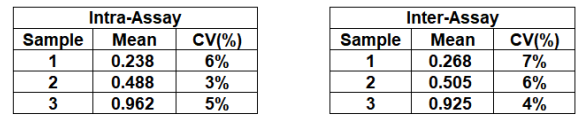
2. All reagents and wash buffer should be used within 12 months from manufacturing date.
3. Before using, bring all components to room temperature (18-25°C). Upon assay completion ensure all components of the kit are returned to appropriate storage conditions.
4. The Substrate is light-sensitive and should be protected from direct sunlight or UV sources.
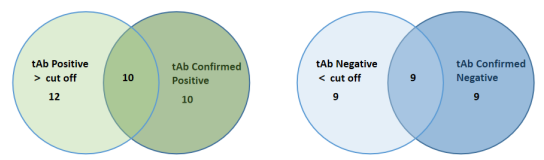
Non-scaled Venn diagram depicting the relationship between total (tAb) and specific confirmed total antibodies in samples. The results are highly consistent, indicating that ELISA has high accuracy in detecting AAV antibodies.
AAV serotype 8 (AAV8) shows a significantly greater liver transduction efficiency than those of other serotypes, which has resulted in efforts to develop this virus as a gene therapy vector for hemophilia A and familial hypercholesterolemia.
One of the major challenges in AAV-based gene therapy is the presence of circulating anti-AAV neutralizing antibodies, which can pre-exist in patients and may prevent successful gene transfer. High levels of circulating anti-AAV neutralizing antibodies can develop after a single administration of gene therapy and can prevent successful gene transfer in patients.
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
| Product Name | Cat. No. | Applications | Host Species | Datasheet | Price | Add to Basket |
|---|
AAV was originally discovered as a contaminating component, and 13 serotypes have been isolated so far: AAV1 to AAV13. AAV belongs to the genus Parvoviridae and is a type of tiny, non-enveloped linear single-stranded DNA virus. Its genome size is about 4700bp and its diameter is 20-26nm. AAV is a defective virus that requires helper viruses (adenovirus helper function genes include E1a, E1b, E2a, E4orf6 and VARNA, herpes virus helper function genes UL5, UL8, UL22, UL9 and regulatory proteins ICP0, ICP4, ICP22), etc. In order to infect host cells and produce new virus particles, when there is no helper virus, wild-type AAV can only integrate into the long arm of chromosome 19 to form a latent infection and replicate along with the host cell chromosome replication. The AAV genome is mainly composed of inverted repeats (ITRs) and 2 open reading frames (ORFs) between the 2 ITRs. The left ORF codes for Rep78, Rep68, Rep52 and Rep40, and the right ORF codes for the 3 capsid proteins VP1 (relative molecular mass 87000), VP2 (relative molecular mass 72000), VP3 (relative molecular mass 62000). The AAV capsid is composed of 60 capsid proteins arranged in an icosahedral structure. Different AAV serotypes have different spatial structures, sequences and tissue specificities of capsid proteins, so the cell surface receptors they recognize and bind to are also very different, which also leads to different tissue types and cell types transfected by different serotypes, and infection efficiency also vary.
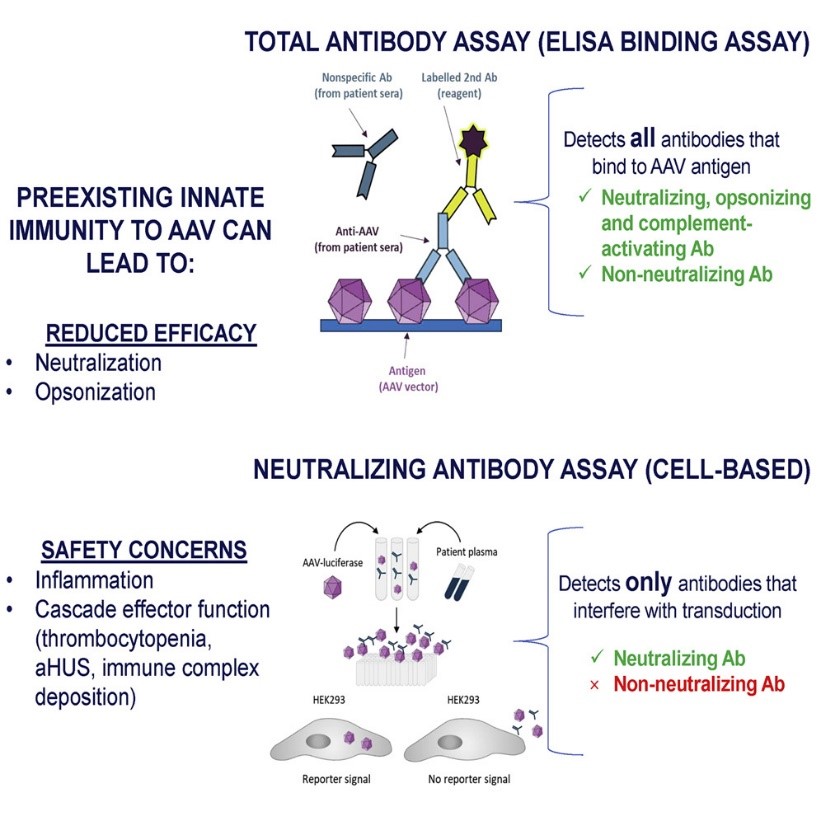 Figure 1. Testing preexisting antibodies prior to AAV gene transfer therapy. (Source: Mendell JR, et al., 2022)
Figure 1. Testing preexisting antibodies prior to AAV gene transfer therapy. (Source: Mendell JR, et al., 2022)
The liver is an organ that has been used for gene therapy earlier. It is now clear that many liver diseases are caused by single gene defects, so it is more suitable for clinical gene therapy than other complex diseases caused by multiple gene defects. The challenges facing liver-targeted gene therapy lie in the effective targeting of liver cells, the stability of the vector genome and sustained high-level expression. The emergence and use of rAAV vectors make it possible to solve this problem. rAAV vectors usually replace the rep and cap genes in wild-type AAV (Wt-AAV) with the target genes for research, leaving only the two ITRs at both ends of the AAV genome. rAAV vectors are currently the most popular vectors. Studies have shown that AAV2, AAV5, AAV8, AAV9 and AAV-DJ are the most commonly used serotypes in the liver. Studies have shown that AAV8, AAV9 and AAV-DJ all have strong affinity for the liver, among which AAV8 It is more hepatotropic and has low immunogenicity and high infection efficiency when injected into living animals. The in vivo hepatocyte transduction efficiency of AAV-DJ is similar to that of AAV8, and it can also show strong infection efficiency in in vitro experiments. Researchers can select appropriate serotypes based on experimental purposes and animal models. Among them, AAV7 and AAV8 have better effects on mouse liver infection; while in human livers, AAV2, AAV5 and AAV8 have stronger affinity.
AAV may cause adverse reactions when conducting clinical experiments on animals and humans. The first is the immune response. When rAAV infects antigen-presenting cells, a mild innate immune response can be induced due to the activation of Toll-like receptor 9 (TLR9). TLR9 recognizes unmethylated CpG in nucleic acids, thereby activating the classical pathway and non-methylated CpG. The classic NF-κB pathway promotes the expression of inflammatory cytokines and interferon response genes. Expression of these genes in turn generates CTL responses and stable neutralizing antibody titers. In order to avoid immune responses caused by AAV-mediated gene transfer, there are currently two commonly used methods: 1, Use immunosuppressive drugs to avoid CTL reactions, which was successful in human factor IX trials; 2, When designing rAAV vectors, DNA sequences that inhibit TLR9, such as multiple copies of TTAGGG from human telomeres, are integrated into the rAAV genome to evade the innate immune response.
Adeno-associated virus serotype 8
References
1. Mendell JR, et al., Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations. Mol Ther Methods Clin Dev. 2022, 25:74-83.
Q: It looks like it is made to detect human anti-AAV8 antibody.
Does it cross-react with mouse serum anti-AAV8 antibodies?
A: DEIASL345 is used to detect human antibodies against AAV8. The antibody detection is done using Anti-Human IgG and cannot detect mouse IgG. Previous testing on monkey samples did not result in any cross-reactivity.
We have a specific assay kit, DEIASL345M, that is designed to detect mouse antibodies and can be recommended.
Successful AAV8 readministration: Suppression of capsid-specific neutralizing antibodies by a combination treatment of bortezomib and CD20 mAb in a mouse model of Pompe disease
J Gene Med
Authors: Choi SJ, Yi JS, Lim JA, Tedder TF, Koeberl DD, Jeck W, Desai AK, Rosenberg A, Sun B, Kishnani PS.
Methods: An AAV8 vector (AAV8-CB-hGAA) that ubiquitously expresses human α-glucosidase was used for initial gene therapy and a second AAV8 vector (AAV8-LSP-hSEAP) that contains a liver-specific promoter to express human secreted embryonic alkaline phosphatase (hSEAP) was used for AAV readministration. Plasma samples were used for determination of anti-AAV8 NAb titers. Cells isolated from whole blood, spleen, and bone marrow were analyzed for B-cell depletion by flow cytometry. The efficiency of AAV readministration was determined by the secretion of hSEAP in blood.
Results: In n?ive mice, an 8-week IS treatment along with AAV8-CB-hGAA injection effectively depleted CD19+ B220+ B cells from blood, spleen, and bone marrow and prevented the formation of anti-AAV8 NAbs. Following administration of AAV8-LSP-hSEAP, increasing levels of hSEAP were detected in blood for up to 6 weeks, indicating successful AAV readministration. In mice pre-immunized with AAV8-CB-hGAA, comparison of IS treatment for 8, 12, 16, and 20 weeks revealed that the 16-week IS treatment demonstrated the highest plasma hSEAP level following AAV8-LSP-hSEAP readministration.
Conclusions: Our data suggest that this combination treatment is an effective IS approach that will allow retreatment of patients with AAV-mediated gene therapy. A combination IS treatment with bortezomib and a mouse-specific CD20 monoclonal antibody effectively suppressed anti-AAV NAbs in naïve mice and in mice with pre-existing antibodies, allowing successful readministration of the same AAV capsid vector.
Split AAV8 Mediated ABCA4 Expression for Gene Therapy of Mouse Stargardt Disease (STGD1)
Hum Gene Ther
Authors: Li R, Jing Q, She K, Wang Q, Jin X, Zhao Q, Su J, Song L, Fu J, Wu X, Xu Q, Lu F, Wei Y, Yang Y
- AAV For Gene Therapy
- Gene Therapy
- GTCDxᵀᴹ Anti-AAV Antibody ELISA Kits
- Liver Tissue-Specific Expression Regulation of AAV
 Anti-AAV Antibody ELISA Kits
Anti-AAV Antibody ELISA Kits
Invoice / Purchase Order
Credit card
![]()