Upregulation of CRMP4, a new prostate cancer metastasis suppressor gene, inhibits tumor growth in a nude mouse intratibial injection model
INTERNATIONAL JOURNAL OF ONCOLOGY
Authors: Zhou, Wei; Xie, Peigen; Pang, Mao; Yang, Bu; Fang, Youqiang; Shu, Tao; Liu, Chang; Wang, Xuan; Zhang, Liangming; Li, Shangfu; Rong, Limin
Abstract
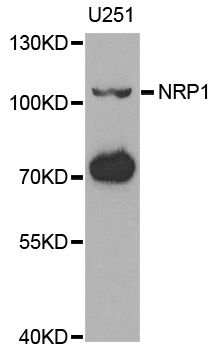
Prostate cancer, the most commonly diagnosed male cancer in North America, has a high incidence of bone metastasis. Our previous study showed collapsin response mediator protein 4 (CRMP4) gene inhibited prostate cancer migration and invasion. In this study, we investigated whether overexpression of CRMP4 gene in prostate cancer cells inhibit tumor bone metastasis. The stable prostate cancer cells overexpressing the CRMP4 gene were constructed using lentivirus infection. Prostate cancer bone metastasis nude mouse model was built though orthotopic prostate implantation, intracardiac injection and intratibial injection with CRMP4 overexpress and control cancer cells. Small animal PET/CT scanning results showed no difference of bone metastatic capacity in orthotopic and intracardiac injection models between CRMP4 overexpression and control group, while CRMP4 overexpression inhibited tumor growth in the intratibial injection model. Moreover, our in vitro study showed CRMP4 overexpression downregulates the Neuropilin1 (NRP1) expression and upregulate the Noggin expression. Immunohistochemical staining of the hind limbs of intratibial injection model was confirmed with cytological experiments. Taken together, our research indicated CRMP4 inhibits prostate cancer cells growth in the nude mouse bone microenvironment and this effect may relate with regulation of NRP1 and Noggin expression.
Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation
JOURNAL OF CLINICAL INVESTIGATION
Authors: Plein, Alice; Calmont, Amelie; Fantin, Alessandro; Denti, Laura; Anderson, Naomi A.; Scambler, Peter J.; Ruhrberg, Christiana
Abstract
In mammals, the outflow tract (OFT) of the developing heart septates into the base of the pulmonary artery and aorta to guide deoxygenated right ventricular blood into the lungs and oxygenated left ventricular blood into the systemic circulation. Accordingly, defective OFT septation is a life-threatening condition that can occur in both syndromic and nonsyndromic congenital heart disease. Even though studies of genetic mouse models have previously revealed a requirement for VEGF-A, the class 3 semaphorin SEMA3C, and their shared receptor neuropilin 1 (NRP1) in OFT development, the precise mechanism by which these proteins orchestrate OFT septation is not yet understood. Here, we have analyzed a complementary set of ligand-specific and tissue-specific mouse mutants to show that neural crest-derived SEMA3C activates NRP1 in the OFT endothelium. Explant assays combined with gene-expression studies and lineage tracing further demonstrated that this signaling pathway promotes an endothelial-to-mesenchymal transition that supplies cells to the endocardial cushions and repositions cardiac neural crest cells (NCCs) within the OFT, 2 processes that are essential for septal bridge formation. These findings elucidate a mechanism by which NCCs cooperate with endothelial cells in the developing OFT to enable the postnatal separation of the pulmonary and systemic circulation.
![]()